Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis
- PMID: 10579718
- PMCID: PMC2169353
- DOI: 10.1083/jcb.147.5.969
Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis
Erratum in
- J Cell Biol 1999 Dec 27;147(7):1585
Abstract
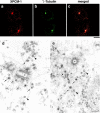
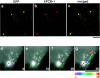
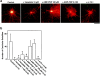
We identified Xenopus pericentriolar material-1 (PCM-1), which had been reported to constitute pericentriolar material, cloned its cDNA, and generated a specific pAb against this molecule. Immunolabeling revealed that PCM-1 was not a pericentriolar material protein, but a specific component of centriolar satellites, morphologically characterized as electron-dense granules, approximately 70-100 nm in diameter, scattered around centrosomes. Using a GFP fusion protein with PCM-1, we found that PCM-1-containing centriolar satellites moved along microtubules toward their minus ends, i.e., toward centrosomes, in live cells, as well as in vitro reconstituted asters. These findings defined centriolar satellites at the molecular level, and explained their pericentriolar localization. Next, to understand the relationship between centriolar satellites and centriolar replication, we examined the expression and subcellular localization of PCM-1 in ciliated epithelial cells during ciliogenesis. When ciliogenesis was induced in mouse nasal respiratory epithelial cells, PCM-1 immunofluorescence was markedly elevated at the apical cytoplasm. At the electron microscopic level, anti-PCM-1 pAb exclusively labeled fibrous granules, but not deuterosomes, both of which have been suggested to play central roles in centriolar replication in ciliogenesis. These findings suggested that centriolar satellites and fibrous granules are identical novel nonmembranous organelles containing PCM-1, which may play some important role(s) in centriolar replication.
Figures
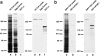



References
-
- Balczon R., Varden C.E., Schroer T.A. Role for microtubules in centrosome doubling in Chinese hamster ovary cells. Cell Motil. Cytoskel. 1999;42:60–72. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

