Enhancement of endothelial cell migration and in vitro tube formation by TAP20, a novel beta 5 integrin-modulating, PKC theta-dependent protein
- PMID: 10579726
- PMCID: PMC2169340
- DOI: 10.1083/jcb.147.5.1073
Enhancement of endothelial cell migration and in vitro tube formation by TAP20, a novel beta 5 integrin-modulating, PKC theta-dependent protein
Abstract
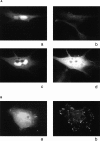
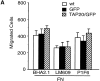
Migration, proliferation, and tube formation of endothelial cells are regulated by a protein kinase C isoenzyme PKCtheta. A full-length cDNA encoding a novel 20-kD protein, whose expression was PKCtheta-dependent, was identified in endothelial cells, cloned, characterized, and designated as theta-associated protein (TAP) 20. Overexpression of TAP20 decreased cell adhesion and enhanced migration on vitronectin and tube formation in three-dimensional culture. An antiintegrin alphavbeta5 antibody prevented these TAP20 effects. Overexpression of TAP20 also decreased focal adhesion formation in alphavbeta3-deficient cells. The interaction between TAP20 and beta5 integrin cytoplasmic domain was demonstrated by protein coprecipitation and immunoblotting. Thus, the discovery of TAP20, which interacts with integrin beta5 and modulates cell adhesion, migration, and tube formation, further defines a possible pathway to angiogenesis dependent on PKCtheta.
Figures











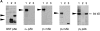
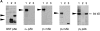
Similar articles
-
Inhibition of protein kinase Calpha prevents endothelial cell migration and vascular tube formation in vitro and myocardial neovascularization in vivo.Circ Res. 2002 Mar 22;90(5):609-16. doi: 10.1161/01.res.0000012503.30315.e8. Circ Res. 2002. PMID: 11909826
-
Regulation of alphaVbeta3 and alphaVbeta5 integrins by dexamethasone in normal human osteoblastic cells.J Cell Biochem. 2000 Mar;77(2):265-76. doi: 10.1002/(sici)1097-4644(20000501)77:2<265::aid-jcb9>3.0.co;2-6. J Cell Biochem. 2000. PMID: 10723092
-
Functional overlap and cooperativity among alphav and beta1 integrin subfamilies during skin angiogenesis.J Invest Dermatol. 2003 Jun;120(6):1100-9. doi: 10.1046/j.1523-1747.2003.12236.x. J Invest Dermatol. 2003. PMID: 12787141
-
Overexpression of protein kinase C-alpha in MCF-7 breast cancer cells results in differential regulation and expression of alphavbeta3 and alphavbeta5.Int J Oncol. 1999 Jul;15(1):127-36. doi: 10.3892/ijo.15.1.127. Int J Oncol. 1999. PMID: 10375605
-
Requirement for protein kinase C theta for cell cycle progression and formation of actin stress fibers and filopodia in vascular endothelial cells.J Biol Chem. 1997 Nov 7;272(45):28704-11. doi: 10.1074/jbc.272.45.28704. J Biol Chem. 1997. PMID: 9353339
Cited by
-
Dichotomous role of integrin-β5 in lung endothelial cells.Pulm Circ. 2022 Oct 1;12(4):e12156. doi: 10.1002/pul2.12156. eCollection 2022 Oct. Pulm Circ. 2022. PMID: 36438452 Free PMC article.
-
The emerging role of protein kinase Cθ in cytoskeletal signaling.J Leukoc Biol. 2013 Mar;93(3):319-27. doi: 10.1189/jlb.0812371. Epub 2012 Nov 27. J Leukoc Biol. 2013. PMID: 23192428 Free PMC article. Review.
-
Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression.Mol Cell Biol. 2004 May;24(9):3992-4003. doi: 10.1128/MCB.24.9.3992-4003.2004. Mol Cell Biol. 2004. PMID: 15082792 Free PMC article.
-
Autologous transplantation of genetically modified iris pigment epithelial cells: a promising concept for the treatment of age-related macular degeneration and other disorders of the eye.Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13090-5. doi: 10.1073/pnas.202486199. Epub 2002 Sep 18. Proc Natl Acad Sci U S A. 2002. PMID: 12239351 Free PMC article.
-
PKCθ-JunB axis via upregulation of VEGFR3 expression mediates hypoxia-induced pathological retinal neovascularization.Cell Death Dis. 2020 May 7;11(5):325. doi: 10.1038/s41419-020-2522-0. Cell Death Dis. 2020. PMID: 32382040 Free PMC article.
References
-
- Chen Y.P., Djaffar I., Pidard D., Steiner B., Cieutat A.M., Caen J.P., Rosa J.P. Ser-752→Pro mutation in the cytoplasmic domain of integrin beta 3 subunit and defective activation of platelet integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc. Natl. Acad. Sci. USA. 1992;89:10169–10173. - PMC - PubMed
-
- Clark E.A., Brugge J.S. Integrins and signal transduction pathwaysthe road taken. Science. 1995;268:233–239. - PubMed
-
- Clark E.A., Hynes R.O. 1997 Keystone symposium on signal transduction by cell adhesion receptors. Biochim. Biophys. Acta. 1997;1333:R9–R16. - PubMed
-
- Crowe D.T., Chiu H., Fong S., Weissman I.L. Regulation of the avidity of integrin alpha 4 beta 7 by the beta 7 cytoplasmic domain. J. Biol. Chem. 1994;269:14411–14418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

