Perlecan maintains the integrity of cartilage and some basement membranes
- PMID: 10579729
- PMCID: PMC2169352
- DOI: 10.1083/jcb.147.5.1109
Perlecan maintains the integrity of cartilage and some basement membranes
Abstract
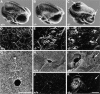
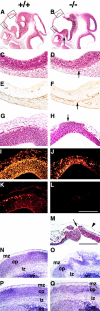
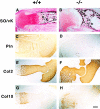
Perlecan is a heparan sulfate proteoglycan that is expressed in all basement membranes (BMs), in cartilage, and several other mesenchymal tissues during development. Perlecan binds growth factors and interacts with various extracellular matrix proteins and cell adhesion molecules. Homozygous mice with a null mutation in the perlecan gene exhibit normal formation of BMs. However, BMs deteriorate in regions with increased mechanical stress such as the contracting myocardium and the expanding brain vesicles showing that perlecan is crucial for maintaining BM integrity. As a consequence, small clefts are formed in the cardiac muscle leading to blood leakage into the pericardial cavity and an arrest of heart function. The defects in the BM separating the brain from the adjacent mesenchyme caused invasion of brain tissue into the overlaying ectoderm leading to abnormal expansion of neuroepithelium, neuronal ectopias, and exencephaly. Finally, homozygotes developed a severe defect in cartilage, a tissue that lacks BMs. The chondrodysplasia is characterized by a reduction of the fibrillar collagen network, shortened collagen fibers, and elevated expression of cartilage extracellular matrix genes, suggesting that perlecan protects cartilage extracellular matrix from degradation.
Figures





References
-
- Aviezer D., Hecht D., Safran M., Eisinger M., David G., Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. - PubMed
-
- Brown J.C., Sasaki T., Göhring W., Yamada Y., Timpl R. The C-terminal domain V of perlecan promotes β1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem. 1997;250:39–46. - PubMed
-
- Castillo G.M., Ngo C., Cummings J., Wight T.N., Snow A.D. Perlecan binds to the β-amyloid proteins (Aβ) of Alzheimer's disease, accelerates Aβ fibril formation, and maintains Aβ fibril stability. J. Neurochem. 1997;69:2452–2465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

