The glucocorticoid receptor is required for stress erythropoiesis
- PMID: 10580006
- PMCID: PMC317156
- DOI: 10.1101/gad.13.22.2996
The glucocorticoid receptor is required for stress erythropoiesis
Abstract
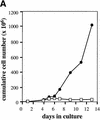
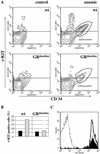
The glucocorticoid receptor (GR) coordinates a multitude of physiological responses in vivo. In vitro, glucocorticoids are required for sustained proliferation of erythroid progenitors (ebls). Here, we analyze the impact of the GR on erythropoiesis in vivo, using GR-deficient mice or mice expressing a GR defective for transactivation. In vitro, sustained proliferation of primary ebls requires an intact GR. In vivo, the GR is required for rapid expansion of ebls under stress situations like erythrolysis or hypoxia. A particular, GR-sensitive progenitor could be identified as being responsible for the stress response. Thus, GR-mediated regulation of ebl proliferation is essential for stress erythropoiesis in vivo.
Figures



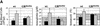
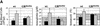

References
-
- Axelrod J, Reisine TD. Stress hormones: Their interaction and regulation. Science. 1984;224:452–459. - PubMed
-
- Bauer A, Ulrich E, Andersson M, Beug H, von Lindern M. Mechanism of transformation by v-ErbA: Replacement of steroid hormone receptor function in self-renewal induction. Oncogene. 1997;15:701–715. - PubMed
-
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: Many actors in search of a plot. Cell. 1995;83:851–857. - PubMed
-
- Beug H, Hayman MJ, Graf T, Benedict SH, Wallbank AM, Vogt PK. S13, a rapidly oncogenic replication-defective avian retrovirus. Virology. 1985;145:141–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
