Nuclear matrix attachment regions antagonize methylation-dependent repression of long-range enhancer-promoter interactions
- PMID: 10580007
- PMCID: PMC317154
- DOI: 10.1101/gad.13.22.3003
Nuclear matrix attachment regions antagonize methylation-dependent repression of long-range enhancer-promoter interactions
Abstract
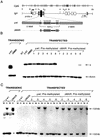
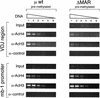
The immunoglobulin intragenic mu enhancer region acts as a locus control region that mediates transcriptional activation over large distances in germ line transformation assays. In transgenic mice, but not in transfected tissue culture cells, the activation of a variable region (V(H)) promoter by the mu enhancer is dependent on flanking nuclear matrix attachment regions (MARs). Here, we examine the effects of DNA methylation, which occurs in early mouse development, on the function of the mu enhancer and the MARs. We find that methylation of rearranged mu genes in vitro, before transfection, represses the ability of the mu enhancer to activate the V(H) promoter over the distance of 1.2 kb. However, methylation does not affect enhancer-mediated promoter activation over a distance of 150 bp. In methylated DNA templates, the mu enhancer alone induces only local chromatin remodeling, whereas in combination with MARs, the mu enhancer generates an extended domain of histone acetylation. These observations provide evidence that DNA methylation impairs the distance independence of enhancer function and thereby imposes a requirement for additional regulatory elements, such as MARs, which facilitate long-range chromatin remodeling.
Figures





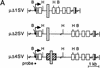
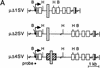
Similar articles
-
Matrix attachment region-dependent function of the immunoglobulin mu enhancer involves histone acetylation at a distance without changes in enhancer occupancy.Mol Cell Biol. 2001 Jan;21(1):196-208. doi: 10.1128/MCB.21.1.196-208.2001. Mol Cell Biol. 2001. PMID: 11113195 Free PMC article.
-
Extension of chromatin accessibility by nuclear matrix attachment regions.Nature. 1997 Jan 16;385(6613):269-72. doi: 10.1038/385269a0. Nature. 1997. PMID: 9000077
-
Dependence of enhancer-mediated transcription of the immunoglobulin mu gene on nuclear matrix attachment regions.Science. 1994 Aug 26;265(5176):1221-5. doi: 10.1126/science.8066460. Science. 1994. PMID: 8066460
-
Combinatorial regulation of transcription II: The immunoglobulin mu heavy chain gene.Immunity. 1995 May;2(5):427-38. doi: 10.1016/1074-7613(95)90024-1. Immunity. 1995. PMID: 7749979 Review. No abstract available.
-
Enhancer selectivity in space and time: from enhancer-promoter interactions to promoter activation.Nat Rev Mol Cell Biol. 2024 Jul;25(7):574-591. doi: 10.1038/s41580-024-00710-6. Epub 2024 Feb 27. Nat Rev Mol Cell Biol. 2024. PMID: 38413840 Free PMC article. Review.
Cited by
-
The human growth hormone locus control region mediates long-distance transcriptional activation independent of nuclear matrix attachment regions.Nucleic Acids Res. 2001 Aug 15;29(16):3356-61. doi: 10.1093/nar/29.16.3356. Nucleic Acids Res. 2001. PMID: 11504873 Free PMC article.
-
Matrix attachment region-dependent function of the immunoglobulin mu enhancer involves histone acetylation at a distance without changes in enhancer occupancy.Mol Cell Biol. 2001 Jan;21(1):196-208. doi: 10.1128/MCB.21.1.196-208.2001. Mol Cell Biol. 2001. PMID: 11113195 Free PMC article.
-
Nucleosome eviction and multiple co-factor binding predict estrogen-receptor-alpha-associated long-range interactions.Nucleic Acids Res. 2014 Jun;42(11):6935-44. doi: 10.1093/nar/gku327. Epub 2014 Apr 29. Nucleic Acids Res. 2014. PMID: 24782518 Free PMC article.
-
A weakened transcriptional enhancer yields variegated gene expression.PLoS One. 2006 Dec 20;1(1):e33. doi: 10.1371/journal.pone.0000033. PLoS One. 2006. PMID: 17183661 Free PMC article.
-
Myc-induced anchorage of the rDNA IGS region to nucleolar matrix modulates growth-stimulated changes in higher-order rDNA architecture.Nucleic Acids Res. 2014 May;42(9):5505-17. doi: 10.1093/nar/gku183. Epub 2014 Mar 7. Nucleic Acids Res. 2014. PMID: 24609384 Free PMC article.
References
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. - PubMed
-
- Bagga R, Emerson BM. An HMG I/Y-containing repressor complex and supercoiled DNA topology are critical for long-range enhancer-dependent transcription in vitro. Genes & Dev. 1997;11:629–639. - PubMed
-
- Belyaev ND, Keohane AM, Turner BM. Histone H4 acetylation and replication timing in Chinese hamster chromosomes. Exp Cell Res. 1996;225:277–285. - PubMed
-
- Bhattacharya SK, Ramchandani S, Cervoni N, Szyf M. A mammalian protein with specific demethylase activity for mCpG DNA. Nature. 1999;397:579–583. - PubMed
-
- Blackwood EM, Kadonaga JT. Going the distance: A current view of enhancer action. Science. 1998;281:61–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
