Altered functional properties of KATP channel conferred by a novel splice variant of SUR1
- PMID: 10581306
- PMCID: PMC2269677
- DOI: 10.1111/j.1469-7793.1999.00337.x
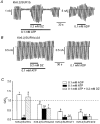
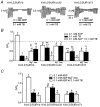
Altered functional properties of KATP channel conferred by a novel splice variant of SUR1
Abstract
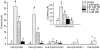
1. ATP-sensitive potassium (KATP) channels are composed of pore-forming (Kir6.x) and regulatory sulphonylurea receptor (SURx) subunits. We have isolated a novel SUR variant (SUR1bDelta33) from a hypothalamic cDNA library. This variant lacked exon 33 and introduced a frameshift that produced a truncated protein lacking the second nucleotide binding domain (NBD2). It was expressed at low levels in hypothalamus, midbrain, heart and the insulin-secreting beta-cell line MIN6. 2. We examined the properties of KATP channels composed of Kir6.2 and SUR1bDelta33 by recording macroscopic currents in membrane patches excised from Xenopus oocytes expressing these subunits. We also investigated the effect of truncating SUR1 at either the start (SUR1bT1) or end (SUR1bT2) of exon 33 on KATP channel properties. 3. Kir6.2/SUR1bDelta33 showed an enhanced open probability (Po = 0.6 at -60 mV) and a reduced ATP sensitivity (Ki, 86 microM), when compared with wild-type channels (Po = 0.3; Ki, 22 microM). However, Kir6.2/SUR1bT1 and Kir6.2/SUR1bT2 resembled the wild-type channel in their Po and ATP sensitivity. 4. Neither MgADP, nor the K+ channel opener diazoxide, enhanced Kir6.2/SUR1bDelta33, Kir6.2/SUR1bT1 or Kir6.2/SUR1bT2 currents, consistent with the idea that these agents require an intact NBD2 for their action. Sulphonylureas blocked KATP channels containing any of the three SUR variants, but in excised patches the extent of block was less than that for the wild-type channel. In intact cells, the extent of sulphonylurea block of Kir6.2/SUR1bDelta33 was greater than that in excised patches and was comparable to that found for wild-type channels. 5. Our results demonstrate that NBD2 is not essential for functional expression or sulphonylurea block, but is required for KATP channel activation by K+ channel openers and nucleotides. Some of the unusual properties of Kir6.2/SUR1bDelta33 resemble those reported for the KATP channel of ventromedial hypothalamic (VMH) neurones, but the fact that this mRNA is expressed at low levels in many other tissues makes it less likely that SUR1bDelta33 serves as the SUR subunit for the VMH KATP channel.
Figures


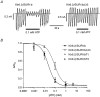

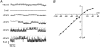
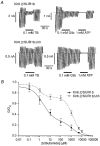
 ), and in the continued presence of azide plus either 0.01 m
), and in the continued presence of azide plus either 0.01 m ) or 0.1 m
) or 0.1 m
References
-
- Aguilar-Bryan L, Bryan A. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocrine Reviews. 1999;20:101–135. - PubMed
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β-cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends in Neurosciences. 1998;21:288–294. - PubMed
-
- Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cellular Signalling. 1990;2:197–214. - PubMed
-
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflügers Archiv. 1990a;415:479–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

