Co-regulation of synaptic efficacy at stable polyneuronally innervated neuromuscular junctions in reinnervated rat muscle
- PMID: 10581308
- PMCID: PMC2269663
- DOI: 10.1111/j.1469-7793.1999.00365.x
Co-regulation of synaptic efficacy at stable polyneuronally innervated neuromuscular junctions in reinnervated rat muscle
Abstract
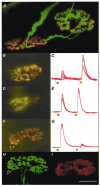
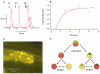
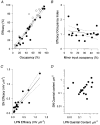
1. Intracellular recordings and quantal analysis of synaptic transmission were made at neuromuscular junctions receiving stable convergent innervation in reinnervated rat lumbrical muscles, following recovery from chronic nerve conduction block. The polyneuronally innervated motor endplates (pi-junctions) were identified by vital staining of lateral plantar nerve (LPN) and sural nerve (SN) motor terminals, using the activity-dependent staining properties of the aminostyryl dyes RH414 and FM1-43, respectively. 2. Endplate depolarisation and quantal content per unit area varied by more than a factor of ten ( approximately 0.1-1. 4 quanta microm-2) between fibres. However, the stable pi-junctions produced nearly equivalent endplate depolarisations and quantal content per unit area, suggesting that synaptic strengths were co-regulated at these motor endplates. Quantal content per unit area was also independent of the size of individual synaptic inputs, or whether one, both or neither input was judged sufficient to produce suprathreshold or subthreshold endplate depolarisations. 3. Simultaneous excitation of convergent LPN and SN inputs from some pi-junctions resulted in profound non-linear summation, and in some cases complete occlusion of the response of the smaller input. The amplitude of the smaller, test responses recovered with a time constant of 2.1 +/- 0.5 ms (mean +/- s.e.m.) on varying the interval between paired stimuli, of similar order to the time constant of repolarisation of the conditioning endplate potential. 4. The data show that it is not necessary for a motor nerve terminal to occupy most of an endplate, or to produce a suprathreshold response in order to become stable. The occlusion of linear summation, similar to that described previously at polyneuronal junctions in neonates, suggests that convergent inputs comprising interdigitated synaptic boutons evoke self-contained synaptic responses at endplates, and that these are non-co-operative with respect to overall endplate depolarisation or safety margin for synaptic transmission.
Figures

Similar articles
-
Persistent polyneuronal innervation in partially denervated rat muscle after reinnervation and recovery from prolonged nerve conduction block.J Neurosci. 1995 Oct;15(10):6327-39. doi: 10.1523/JNEUROSCI.15-10-06327.1995. J Neurosci. 1995. PMID: 7472398 Free PMC article.
-
Calcium channels coupled to neurotransmitter release at dually innervated neuromuscular junctions in the newborn rat.Neuroscience. 2001;102(3):697-708. doi: 10.1016/s0306-4522(00)00507-8. Neuroscience. 2001. PMID: 11226706
-
Activity-dependent and -independent synaptic interactions during reinnervation of partially denervated rat muscle.J Physiol. 1988 Jul;401:53-75. doi: 10.1113/jphysiol.1988.sp017151. J Physiol. 1988. PMID: 3171995 Free PMC article.
-
[A reminder of the structure and function of the skeletal neuromuscular junction].Ann Dermatol Venereol. 2009 May;136 Suppl 4:S55-60. doi: 10.1016/S0151-9638(09)74528-4. Ann Dermatol Venereol. 2009. PMID: 19576486 Review. French.
-
Spatial versus consumptive competition at polyneuronally innervated neuromuscular junctions.Exp Physiol. 1994 Jul;79(4):465-94. doi: 10.1113/expphysiol.1994.sp003781. Exp Physiol. 1994. PMID: 7946277 Review. No abstract available.
Cited by
-
Segmentation of the mouse fourth deep lumbrical muscle connectome reveals concentric organisation of motor units.J Physiol. 2013 Oct 1;591(19):4859-75. doi: 10.1113/jphysiol.2013.258087. Epub 2013 Aug 12. J Physiol. 2013. PMID: 23940381 Free PMC article.
-
Endomicroscopy and electromyography of neuromuscular junctions in situ.Ann Clin Transl Neurol. 2014 Nov;1(11):867-83. doi: 10.1002/acn3.124. Epub 2014 Oct 10. Ann Clin Transl Neurol. 2014. PMID: 25540801 Free PMC article.
-
NMJ-morph reveals principal components of synaptic morphology influencing structure-function relationships at the neuromuscular junction.Open Biol. 2016 Dec;6(12):160240. doi: 10.1098/rsob.160240. Open Biol. 2016. PMID: 27927794 Free PMC article.
-
Confocal Endomicroscopy of Neuromuscular Junctions Stained with Physiologically Inert Protein Fragments of Tetanus Toxin.Biomolecules. 2021 Oct 12;11(10):1499. doi: 10.3390/biom11101499. Biomolecules. 2021. PMID: 34680132 Free PMC article.
-
Disparity in neurotransmitter release probability among competing inputs during neuromuscular synapse elimination.J Neurosci. 2000 Dec 1;20(23):8771-9. doi: 10.1523/JNEUROSCI.20-23-08771.2000. J Neurosci. 2000. PMID: 11102485 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

