Muscarinic receptor heterogeneity in follicle-enclosed Xenopus oocytes
- PMID: 10581312
- PMCID: PMC2269680
- DOI: 10.1111/j.1469-7793.1999.00409.x
Muscarinic receptor heterogeneity in follicle-enclosed Xenopus oocytes
Abstract
1. Ionic current responses elicited by acetylcholine (ACh) in follicle-enclosed Xenopus oocytes (follicles) were studied using the two-electrode voltage-clamp technique. ACh generated a fast chloride current (Fin) and inhibited K+ currents gated by cAMP (IK,cAMP) following receptor activation by adenosine, follicle-stimulating hormone or noradrenaline. These previously described cholinergic responses were confirmed to be of the muscarinic type, and were independently generated among follicles from different frogs. 2. Inhibition of IK,cAMP was about 100 times more sensitive to ACh than Fin activation; the half-maximal effective concentrations (EC50) were 6.6 +/- 0.4 and 784 +/- 4 nM, respectively. 3. Both responses were blocked by several muscarinic receptor antagonists. Using the respective EC50 concentrations of ACh as standard, the antagonist 4-diphenylacetoxy-N-methylpiperidine methiodide blocked the two effects with very different potencies. Fin was blocked with a half-maximal inhibitory concentration (IC50) of 2.4 +/- 0.07 nM, whilst the IC50 for IK,cAMP inhibition was 5.9 +/- 0.2 microM. 4. Oxotremorine, a muscarinic agonist, preferentially stimulated IK, cAMP inhibition (EC50 = 15.8 +/- 1.4 microM), whilst Fin was only weakly activated. In contrast, oxotremorine inhibited Fin generated by ACh with an IC50 of 2.3 +/- 0.7 microM. 5. Fin elicited via purinergic receptor stimulation was not affected by oxotremorine, indicating that the inhibition produced was specific to the muscarinic receptor, and suggesting that muscarinic actions do not exert a strong effect on follicular cell-oocyte coupling. 6. Using reverse transcription-PCR, transcripts of a previously cloned muscarinic receptor from Xenopus (XlmR) were amplified from the RNA of both the isolated follicular cells and the oocyte. The pharmacological and molecular characteristics suggest that XlmR is involved in IK,cAMP inhibition. 7. In conclusion, follicular cells possess two different muscarinic receptors, one resembling the M2 (or M4) subtype and the other the M3 subtype. These receptors are coupled to distinct membrane mechanisms leading to independent regulation of two membrane conductances.
Figures




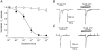

Similar articles
-
Novel Cl- currents elicited by follicle stimulating hormone and acetylcholine in follicle-enclosed Xenopus oocytes.J Gen Physiol. 1993 Nov;102(5):833-57. doi: 10.1085/jgp.102.5.833. J Gen Physiol. 1993. PMID: 8301259 Free PMC article.
-
Epithelium and/or theca are required for ATP-elicited K+ current in follicle-enclosed Xenopus oocytes.J Cell Physiol. 2005 Mar;202(3):814-21. doi: 10.1002/jcp.20184. J Cell Physiol. 2005. PMID: 15389645
-
Characterization of functional effects of Z-338, a novel gastroprokinetic agent, on the muscarinic M1, M2, and M3 receptors expressed in Xenopus oocytes.Eur J Pharmacol. 2004 Nov 28;505(1-3):31-5. doi: 10.1016/j.ejphar.2004.10.003. Eur J Pharmacol. 2004. PMID: 15556134
-
Pharmacology and second messenger interactions of cloned muscarinic receptors.Biochem Pharmacol. 1991 Oct 9;42(9):1645-53. doi: 10.1016/0006-2952(91)90498-t. Biochem Pharmacol. 1991. PMID: 1930292 Review. No abstract available.
-
Molecular probes for muscarinic receptors: functionalized congeners of selective muscarinic antagonists.Life Sci. 1995;56(11-12):823-30. doi: 10.1016/0024-3205(95)00016-y. Life Sci. 1995. PMID: 10188781 Free PMC article. Review.
Cited by
-
Functional interaction between native G protein-coupled purinergic receptors in Xenopus follicles.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16680-5. doi: 10.1073/pnas.0905811106. Epub 2009 Sep 10. Proc Natl Acad Sci U S A. 2009. PMID: 19805357 Free PMC article.
-
Activation of volume-regulated Cl(-) channels by ACh and ATP in Xenopus follicles.J Physiol. 2000 Jun 15;525 Pt 3(Pt 3):721-34. doi: 10.1111/j.1469-7793.2000.00721.x. J Physiol. 2000. PMID: 10856124 Free PMC article.
References
-
- Adashi EY, Hsueh AJ. Stimulation of β2-adrenergic responsiveness by follicle-stimulating hormone in rat granulosa cells in vitro and in vivo. Endocrinology. 1981;108:2170–2178. - PubMed
-
- Arellano RO, Garay E, Miledi R. Cl− currents activated via purinergic receptors in Xenopus follicles. American Journal of Physiology. 1998;274:C333–340. - PubMed
-
- Arellano RO, Woodward RM, Miledi R. Ion channels and membrane receptors in follicle-enclosed Xenopus oocytes. In: Narahashi T, editor. Ion Channels. Vol. 4. New York: Plenum Press; 1996. pp. 203–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

