Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons
- PMID: 10581316
- PMCID: PMC2269673
- DOI: 10.1111/j.1469-7793.1999.00467.x
Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons
Abstract
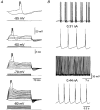
1. Electrophysiological recordings and pharmacological manipulations were used to investigate the mechanisms underlying the generation of action potential burst firing and its postsynaptic consequences in visually identified rat layer 5 pyramidal neurons in vitro. 2. Based upon repetitive firing properties and subthreshold membrane characteristics, layer 5 pyramidal neurons were separated into three classes: regular firing and weak and strong intrinsically burst firing. 3. High frequency (330 +/- 10 Hz) action potential burst firing was abolished or greatly weakened by the removal of Ca2+ (n = 5) from, or by the addition of the Ca2+ channel antagonist Ni2+ (250-500 microm; n = 8) to, the perfusion medium. 4. The blockade of apical dendritic sodium channels by the local dendritic application of TTX (100 nM; n = 5) abolished or greatly weakened action potential burst firing, as did the local apical dendritic application of Ni2+ (1 mM; n = 5). 5. Apical dendritic depolarisation resulted in low frequency (157 +/- 26 Hz; n = 6) action potential burst firing in regular firing neurons, as classified by somatic current injection. The intensity of action potential burst discharges in intrinsically burst firing neurons was facilitated by dendritic depolarisation (n = 11). 6. Action potential amplitude decreased throughout a burst when recorded somatically, suggesting that later action potentials may fail to propagate axonally. Axonal recordings demonstrated that each action potential in a burst is axonally initiated and that no decrement in action potential amplitude is apparent in the axon > 30 microm from the soma. 7. Paired recordings (n = 16) from synaptically coupled neurons indicated that each action potential in a burst could cause transmitter release. EPSPs or EPSCs evoked by a presynaptic burst of action potentials showed use-dependent synaptic depression. 8. A postsynaptic, TTX-sensitive voltage-dependent amplification process ensured that later EPSPs in a burst were amplified when generated from membrane potentials positive to -60 mV, providing a postsynaptic mechanism that counteracts use-dependent depression at synapses between layer 5 pyramidal neurons.
Figures










References
-
- Amitai Y, Friedman A, Connors BW, Gutnick MJ. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cerebral Cortex. 1993;3:26–38. - PubMed
-
- Ball GJ, Gloor P, Schaul N. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalography and Clinical Neurophysiology. 1977;43:346–361. - PubMed
-
- Bekkers JM, Stuart G. Distribution and properties of potassium channels in the soma and apical dendrites of layer 5 cortical pyramidal neurons. Society for Neuroscience Abstracts. 1998;24:2019–807.8.
-
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. Journal of Comparative Neurology. 1990;296:598–613. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

