Dynamic Ca2+ signalling in rat arterial smooth muscle cells under the control of local renin-angiotensin system
- PMID: 10581318
- PMCID: PMC2269666
- DOI: 10.1111/j.1469-7793.1999.00497.x
Dynamic Ca2+ signalling in rat arterial smooth muscle cells under the control of local renin-angiotensin system
Abstract
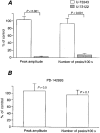
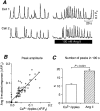
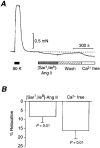
1. We visualized the changes in intracellular Ca2+ concentration ([Ca2+]i), using fluo-3 as an indicator, in individual smooth muscle cells within intact rat tail artery preparations. 2. On average in about 45 % of the vascular smooth muscle cells we found spontaneous Ca2+ waves and oscillations ( approximately 0.13 Hz), which we refer to here as Ca2+ ripples because the peak amplitude of [Ca2+]i was about one-seventh of that of Ca2+ oscillations evoked by noradrenaline. 3. We also found another pattern of spontaneous Ca2+ transients often in groups of two to three cells. They were rarely observed and are referred to as Ca2+ flashes because their peak amplitude was nearly twice as large as that in noradrenaline-evoked responses. 4. Sympathetic nerve activity was not considered responsible for the Ca2+ ripples, and they were abolished by inhibitors of either the Ca2+ pump in the sarcoplasmic reticulum (cyclopiazonic acid) or phospholipase C (U-73122). 5. Both angiotensin antagonists ([Sar1,Ile8]-angiotensin II and losartan) and an angiotensin converting enzyme inhibitor (captopril) inhibited the Ca2+ ripples. 6. The extracellular Ca2+-dependent tension borne by unstimulated arterial rings was reduced by the angiotensin antagonist by approximately 50 %. 7. These results indicate that the Ca2+ ripples are generated via inositol 1,4, 5-trisphosphate-induced Ca2+ release from the intracellular Ca2+ stores in response to locally produced angiotensin II, which contributes to the maintenance of vascular tone.
Figures





References
-
- Baker KM, Booz GW, Dostal DE. Cardiac actions of angiotensin II: Role of an intracardiac renin-angiotensin system. Annual Review of Physiology. 1992;54:227–241. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Brinson AE, Harding T, Diliberto PA, He Y, Li X, Hunter D, Herman B, Earp HS, Graves LM. Regulation of a calcium-dependent tyrosine kinase in vascular smooth muscle cells by angiotensin II and platelet-derived growth factor. Dependence on calcium and the actin cytoskeleton. Journal of Biological Chemistry. 1998;273:1711–1718. - PubMed
-
- Cody WL, Doherty AM, He JX, DePue PL, Rapundalo ST, Hingorani GA, Major TC, Panek RL, Dudley DT, Haleen SJ, LaDouceuer D, Hill KE, Flynn MA, Reynolds EE. Design of a functional hexapeptide antagonist of endothelin. Journal of Medical Chemistry. 1992;35:3301–3303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

