Pharmacokinetics of cefepime in patients with thermal burn injury
- PMID: 10582870
- PMCID: PMC89575
- DOI: 10.1128/AAC.43.12.2848
Pharmacokinetics of cefepime in patients with thermal burn injury
Abstract
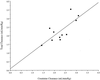
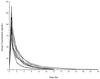
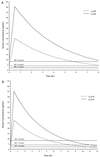
The pharmacokinetics of cefepime following administration of a single 2-g dose were evaluated for 12 adult patients with thermal burn injury and suspected or documented infection. Serial blood and urine samples for cefepime concentration determination were obtained for 24 h following drug administration. Serum and urine cefepime concentrations were determined by high-performance liquid chromatography and serum concentrations were fit to a two-compartment pharmacokinetic model. Mean (standard deviation [SD]) age, actual body weight (ABW), percent total body surface area burned, and days postburn at the time of study were 41 (13) years, 84 (22) kg, 36 (17)%, and 9 (3) days, respectively. Mean (SD) measured creatinine clearance (CL(CR)), total clearance (CL(T)), renal clearance (CL(R)), alpha phase half-life, beta phase half-life, and volume of distribution at steady state (V(SS)) were 135 (31) ml/min, 8.8 (2.4) liters/h, 8.1 (2.0) liters/h, 0.33 (0.14) h, 2.8 (0.6) h, and 0.43 (0.10) liters/kg ABW, respectively. Cefepime CL(T) and CL(R) in burn patients were similar to previously reported values for healthy volunteers when normalized by CL(CR). Stepwise multiple regression was used to associate CL(T) with CL(CR) and days postburn (r(2) = 0.861), CL(R) with CL(CR) and days postburn (r(2) = 0.773), nonrenal clearance with percent third-degree (% 3 degrees ) burn and albumin concentration (r(2) = 0.550), and V(SS) only with % 3 degrees burn (r(2) = 0.624). Simulated steady-state serum concentrations obtained by using the patients' pharmacokinetic parameters exceeded the susceptibility interpretive standard (breakpoint) of cefepime for at least 60% of the dosing interval with dosing regimens of 1 g every 8 h (q8h), 2 g q8h, and 2 g q12h. Despite differences in pharmacokinetic parameters between our patients and healthy volunteers, it appears that these dosing regimens may be adequate in similar burn patients.
Figures
References
-
- Adam D, Zellner P R, Koeppe P, Wesch R. Pharmacokinetics of ticarcillin/clavulanate in severely burned patients. J Antimicrob Chemother. 1989;24(Suppl. B):121–130. - PubMed
-
- Bailie G R, Ackerman B H, Fischer J, Solem L D, Rotschafer J C. Increased vancomycin dosage requirements in young burn patients. J Burn Care Rehabil. 1984;5:376–378.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

