Hydroxyurea potentiates the antiherpesvirus activities of purine and pyrimidine nucleoside and nucleoside phosphonate analogs
- PMID: 10582877
- PMCID: PMC89582
- DOI: 10.1128/AAC.43.12.2885
Hydroxyurea potentiates the antiherpesvirus activities of purine and pyrimidine nucleoside and nucleoside phosphonate analogs
Abstract
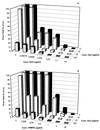
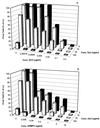
Hydroxyurea has been shown to potentiate the anti-human immunodeficiency virus activities of 2',3'-dideoxynucleoside analogs such as didanosine. We have now evaluated in vitro the effect of hydroxyurea on the antiherpesvirus activities of several nucleoside analogs (acyclovir [ACV], ganciclovir [GCV], penciclovir [PCV], lobucavir [LBV], (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine [H2G], and brivudin and nucleoside phosphonate analogs (cidofovir [CDV] and adefovir [ADV]). When evaluated in cytopathic effect (CPE) reduction assays, hydroxyurea by itself had little effect on CPE progression and potentiated in a subsynergistic (herpes simplex virus type 1 [HSV-1]) to synergistic (HSV-2) fashion the antiviral activities of ACV, GCV, PCV, LBV, H2G, ADV, and CDV. Hydroxyurea also caused marked increases in the activities of ACV, GCV, PCV, LBV, and H2G (compounds that depend for their activation on a virus-encoded thymidine kinase [TK]) against TK-deficient (TK(-)) HSV-1. In fact, in combination with hydroxyurea the 50% effective concentrations of these compounds for inhibition of TK(-) HSV-1-induced CPE decreased from values of 20 to > or = 100 microg/ml (in the absence of hydroxyurea) to values of 1 to 5 microg/ml (in the presence of hydroxyurea at 25 to 100 microg/ml). When evaluated in a single-cycle virus yield reduction assay, hydroxyurea at a concentration of 100 microg/ml inhibited progeny virus production by 60 to 90% but had little effect on virus yield at a concentration of 25 microg/ml. Under these assay conditions hydroxyurea still elicited a marked potentiating effect on the antiherpesvirus activities of GCV and CDV, but this effect was less pronounced than that in the CPE reduction assay. It is conceivable that the potentiating effect of hydroxyurea stems from a depletion of the intracellular deoxynucleoside triphosphate pools, thus favoring the triphosphates of the nucleoside analogues (or the diphosphates of the nucleoside phosphonate analogues) in their competition with the natural nucleotides at the viral DNA polymerase level. The possible clinical implications of these findings are discussed.
Figures
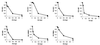
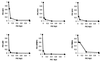
Similar articles
-
The A167Y mutation converts the herpes simplex virus type 1 thymidine kinase into a guanosine analogue kinase.Biochemistry. 2002 May 21;41(20):6517-24. doi: 10.1021/bi0255930. Biochemistry. 2002. PMID: 12009916
-
The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo.Antimicrob Agents Chemother. 1998 Feb;42(2):216-22. doi: 10.1128/AAC.42.2.216. Antimicrob Agents Chemother. 1998. PMID: 9527762 Free PMC article.
-
The antiherpesvirus activity of H2G [(R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine] is markedly enhanced by the novel immunosuppressive agent mycophenolate mofetil.Antimicrob Agents Chemother. 1998 Dec;42(12):3285-9. doi: 10.1128/AAC.42.12.3285. Antimicrob Agents Chemother. 1998. PMID: 9835529 Free PMC article.
-
Bicyclic pyrimidine nucleoside analogues (BCNAs) as highly selective and potent inhibitors of varicella-zoster virus replication.J Antimicrob Chemother. 2002 Jul;50(1):5-9. doi: 10.1093/jac/dkf037. J Antimicrob Chemother. 2002. PMID: 12096000 Review.
-
Chemotherapy of varicella-zoster virus by a novel class of highly specific anti-VZV bicyclic pyrimidine nucleosides.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):287-95. doi: 10.1016/s0925-4439(02)00091-1. Biochim Biophys Acta. 2002. PMID: 12084470 Review.
Cited by
-
Ribonucleotide reductase inhibitors hydroxyurea, didox, and trimidox inhibit human cytomegalovirus replication in vitro and synergize with ganciclovir.Antiviral Res. 2013 Oct;100(1):151-8. doi: 10.1016/j.antiviral.2013.07.016. Epub 2013 Aug 6. Antiviral Res. 2013. PMID: 23933116 Free PMC article.
-
Advances in the Development of Antiviral Strategies against Parvovirus B19.Viruses. 2019 Jul 18;11(7):659. doi: 10.3390/v11070659. Viruses. 2019. PMID: 31323869 Free PMC article. Review.
References
-
- Baba M, Pauwels R, Balzarini J, Herdewijn P, De Clercq E, Desmyter J. Ribavirin antagonizes inhibitory effects of pyrimidine 2′,3′-dideoxynucleosides but enhances inhibitory effects of purine 2′,3′-dideoxynucleosides on replication of human immunodeficiency virus in vitro. Antimicrob Agents Chemother. 1987;31:1613–1617. - PMC - PubMed
-
- Balzarini J, De Clercq E. 5-Phosphoribosyl 1-pyrophosphate synthetase converts the acyclic nucleoside phosphonates 9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine and 9-(2-phosphonylmethoxyethyl)adenine directly to their antivirally active diphosphate derivatives. J Biol Chem. 1991;266:8686–8689. - PubMed
-
- Biron F, Lucht F, Peyramond D, Fresard A, Vallet T, Nugier F, Grange J, Malley S, Hamedi-Sangsari F, Vila J. Anti-HIV activity of the combination of didanosine and hydroxyurea in HIV-1-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:36–40. - PubMed
-
- Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol. 1997;34:15–21. - PubMed
-
- De Clercq E. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpesviruses and retroviruses. Rev Med Virol. 1995;5:149–164.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

