Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activities by the C-8-methoxy group
- PMID: 10582891
- PMCID: PMC89596
- DOI: 10.1128/AAC.43.12.2969
Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activities by the C-8-methoxy group
Abstract
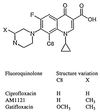
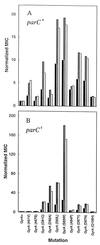
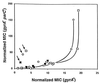
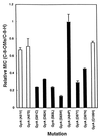
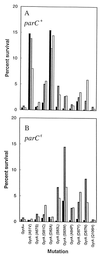
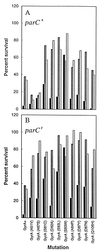
Antibacterial activities of gatifloxacin (AM1155), a new C-8-methoxy fluoroquinolone, and two structurally related compounds, AM1121 and ciprofloxacin, were studied with an isogenic set of ten quinolone-resistant, gyrA (gyrase) mutants of Escherichia coli. To compare the effect of each mutation on resistance, the mutant responses were normalized to those of wild-type cells. Alleles exhibiting the most resistance to growth inhibition mapped in alpha-helix 4, which is thought to lie on a GyrA dimer surface that interacts with DNA. The C-8-methoxy group lowered the resistance due to these mutations more than it lowered resistance arising from several gyrA alleles located outside alpha-helix 4. These data are consistent with alpha-helix 4 being a distinct portion of the quinolone-binding site of GyrA. A helix change to proline behaved more like nonhelix alleles, indicating that helix perturbation differs from the other changes at helix residues. Addition of a parC (topoisomerase IV) resistance allele revealed that the C-8-methoxy group also facilitated attack of topoisomerase IV. When lethal effects were measured at a constant multiple of the minimum inhibitory concentration for each fluoroquinolone to normalize for differences in bacteriostatic action, gatifloxacin was more potent than the C-8-H compounds, both in the presence and absence of protein synthesis (an exception was observed when alanine was substituted for aspartic acid at position 82). Collectively, these data show that the C-8-methoxy group contributes to the enhanced activity of gatifloxacin against resistant gyrase and wild-type topoisomerase IV.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

