Stereoselective pharmacokinetics of ketoprofen and ketoprofen glucuronide in end-stage renal disease: evidence for a 'futile cycle' of elimination
- PMID: 10583018
- PMCID: PMC2014373
- DOI: 10.1046/j.1365-2125.1999.00046.x
Stereoselective pharmacokinetics of ketoprofen and ketoprofen glucuronide in end-stage renal disease: evidence for a 'futile cycle' of elimination
Abstract
Aims: To assess if futile cycling of ketoprofen occurs in patients with decreased renal function.
Methods: Ketoprofen was administered to six haemodialysis-dependent patients with end-stage renal disease as single (50 mg) or multiple doses (50 mg three times daily, for 7 days). Plasma and dialysate concentrations of the unconjugated and glucuronidated R- and S-enantiomers of ketoprofen were determined using h.p.l.c. following the single and multiple dosing.
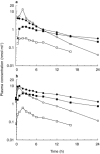
Results: The oral clearance was decreased and terminal elimination half-lives of R- and S-ketoprofen and the corresponding acyl glucuronides were increased in functionally anephric patients compared with healthy subjects. In contrast with the R-isomers, S-ketoprofen and S-ketoprofen glucuronide exhibited an unexpected accumulation (2.7-3. 8 fold) after repeated dosing achieving S:R ratios of 3.3+/-1.7 and 11.2+/-5.3, respectively. The plasma dialysis clearances for R- and S-ketoprofen glucuronides were 49.4+/-19.8 and 39.0+/-15.9 ml min-1, respectively, and 10.8+/-17.6 and 13.3+/-23.5 ml min-1 for unconjugated R- and S-ketoprofen.
Conclusions: The selective accumulation of S-ketoprofen and its acyl glucuronide are consistent with amplification of chiral inversion subsequent to futile cycling between R-ketoprofen and R-ketoprofen glucuronide. Severe renal insufficiency, and possibly more modest decrements, results in a disproportionate increase in systemic exposure to the S-enantiomer which inhibits both pathologic and homeostatic prostaglandin synthesis.
Figures
Similar articles
-
Stereoselective Pharmacokinetics of Ketoprofen After Oral Administration of Modified-Release Formulations in Caucasian Healthy Subjects.Eur J Drug Metab Pharmacokinet. 2016 Dec;41(6):787-793. doi: 10.1007/s13318-015-0313-2. Eur J Drug Metab Pharmacokinet. 2016. PMID: 26590950 Clinical Trial.
-
The influence of renal function on the pharmacokinetics of unchanged and acyl-glucuroconjugated ketoprofen enantiomers after 50 and 100 mg racemic ketoprofen.Br J Clin Pharmacol. 1996 Aug;42(2):163-9. doi: 10.1046/j.1365-2125.1996.03864.x. Br J Clin Pharmacol. 1996. PMID: 8864313 Free PMC article. Clinical Trial.
-
Pharmacokinetics of ketoprofen enantiomers after intravenous administration of racemate in camels: effect of gender.J Vet Pharmacol Ther. 2000 Jun;23(3):137-43. doi: 10.1046/j.1365-2885.2000.00264.x. J Vet Pharmacol Ther. 2000. PMID: 11110100
-
Clinical pharmacokinetics of dexketoprofen.Clin Pharmacokinet. 2001;40(4):245-62. doi: 10.2165/00003088-200140040-00002. Clin Pharmacokinet. 2001. PMID: 11368291 Review.
-
Preclinical and clinical development of dexketoprofen.Drugs. 1996;52 Suppl 5:24-45; discussion 45-6. doi: 10.2165/00003495-199600525-00005. Drugs. 1996. PMID: 8922555 Review.
Cited by
-
Physiologically-based pharmacokinetic modeling for absorption, transport, metabolism and excretion.J Pharmacokinet Pharmacodyn. 2010 Dec;37(6):591-615. doi: 10.1007/s10928-010-9185-x. Epub 2010 Dec 14. J Pharmacokinet Pharmacodyn. 2010. PMID: 21153869 Review.
-
Inhibition of human drug-metabolising cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in vitro by uremic toxins.Eur J Clin Pharmacol. 2014 Sep;70(9):1097-106. doi: 10.1007/s00228-014-1709-7. Epub 2014 Jun 24. Eur J Clin Pharmacol. 2014. PMID: 24954688
-
Stereoselective Pharmacokinetics of Ketoprofen After Oral Administration of Modified-Release Formulations in Caucasian Healthy Subjects.Eur J Drug Metab Pharmacokinet. 2016 Dec;41(6):787-793. doi: 10.1007/s13318-015-0313-2. Eur J Drug Metab Pharmacokinet. 2016. PMID: 26590950 Clinical Trial.
-
Pharmacokinetics and dosage adjustment in patients with renal dysfunction.Eur J Clin Pharmacol. 2009 Aug;65(8):757-73. doi: 10.1007/s00228-009-0678-8. Epub 2009 Jun 20. Eur J Clin Pharmacol. 2009. PMID: 19543887 Review.
-
Stereoselective binding of mexiletine and ketoprofen enantiomers with human serum albumin domains.Acta Pharmacol Sin. 2012 May;33(5):710-6. doi: 10.1038/aps.2012.8. Acta Pharmacol Sin. 2012. PMID: 22555373 Free PMC article.
References
-
- Murray MD, Brater DC. Nonsteroidal anti-inflammatory drugs. Clin Ger Med. 1990;6:365–397. - PubMed
-
- Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal anti-inflammatory drugs. N Engl J Med. 1984;310:563–572. - PubMed
-
- Murray MD, Black PK, Kuzmik DD, et al. Acute and chronic effects of nonsteroidal antiinflammatory drugs on glomerular filtration rate in elderly patients. Am J Med Sci. 1995;310:188–197. - PubMed
-
- Suesa N, Fernandez MF, Gutierrez M, et al. Stereoselective cyclooxygenase inhibition in cellular models by the enantiomers of ketoprofen. Chirality. 1993;5:589–595. - PubMed
-
- Aberg G, Ciofalo VB, Pendleton RG, Ray G, Weddle D. Inversion of (R)- to (S)-ketoprofen in eight animal species. Chirality. 1995;7:383–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

