The effect of dosing regimen on the pharmacokinetics of risedronate
- PMID: 10583024
- PMCID: PMC2014385
- DOI: 10.1046/j.1365-2125.1999.00035.x
The effect of dosing regimen on the pharmacokinetics of risedronate
Abstract
Aims: To examine the effect of timing of a risedronate dose relative to food intake on the rate and extent of risedronate absorption following single-dose, oral administration to healthy male and female volunteers.
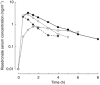
Methods: A single-dose, randomized, parallel study design was conducted with volunteers assigned to four treatment groups (31 or 32 subjects per group, 127 subjects total). Each subject was orally administered 30 mg risedronate. Group 1 was fasted for 10 h prior to and 4 h after dosing (fasted group); Groups 2 and 3 were fasted for 10 h and were dosed 1 and 0.5 h, respectively, before a high-fat breakfast; and Group 4 was dosed 2 h after a standard dinner. Blood and urine samples were collected for 168 h after dosing. Pharmacokinetic parameters were estimated by simultaneous analysis of risedronate serum concentration and urinary excretion rate-time data.
Results: Extent of risedronate absorption (AUC and Ae ) was comparable (P=0.4) in subjects dosed 2 h after dinner and 0.5 h before breakfast; however, a significantly greater extent of absorption occurred when risedronate was given 1 or 4 h prior to a meal (1.4- to 2.3-fold greater). Administration 0.5, 1, or 4 h prior to a meal resulted in a significantly greater rate of absorption (Cmax 2.8-, 3.5-, and 4.1-fold greater, respectively) when compared with 2 h after dinner.
Conclusions: The comparable extent of risedronate absorption when administered either 0.5-1 h before breakfast or 2 h after an evening meal support previous clinical studies where risedronate was found to have similar effectiveness using these dosing regimens. This flexibility in the timing of risedronate administration may provide patients an alternative means to achieve the desired efficacy while maintaining their normal daily routine.
Figures

Similar articles
-
Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease.Drugs. 2001;61(5):685-712. doi: 10.2165/00003495-200161050-00013. Drugs. 2001. PMID: 11368289 Review.
-
Risedronate gastrointestinal absorption is independent of site and rate of administration.Pharm Res. 1998 Feb;15(2):228-32. doi: 10.1023/a:1011910517200. Pharm Res. 1998. PMID: 9523308 Clinical Trial.
-
Clinical trial of risedronate in Japanese volunteers: a study on the effects of timing of dosing on absorption.J Bone Miner Metab. 2004;22(2):120-6. doi: 10.1007/s00774-003-0459-x. J Bone Miner Metab. 2004. PMID: 14999522 Clinical Trial.
-
Dose-proportional pharmacokinetics of risedronate on single-dose oral administration to healthy volunteers.J Clin Pharmacol. 2000 Mar;40(3):258-65. doi: 10.1177/00912700022008928. J Clin Pharmacol. 2000. PMID: 10709154 Clinical Trial.
-
[Episcleritis secondary to risedronate].Med Clin (Barc). 2002 Apr 27;118(15):598-9. doi: 10.1016/s0025-7753(02)72464-0. Med Clin (Barc). 2002. PMID: 12015954 Review. Spanish. No abstract available.
Cited by
-
Optimal Dosing Regimen of Osteoporosis Drugs in Relation to Food Intake as the Key for the Enhancement of the Treatment Effectiveness-A Concise Literature Review.Foods. 2021 Mar 29;10(4):720. doi: 10.3390/foods10040720. Foods. 2021. PMID: 33805435 Free PMC article. Review.
-
Urinary excretion: does it accurately reflect relative differences in bioavailability/systemic exposure when renal clearance is nonlinear?Pharm Res. 2004 May;21(5):781-4. doi: 10.1023/b:pham.0000026428.48103.4f. Pharm Res. 2004. PMID: 15180334
-
An open-label randomized study of the relative absorption of gastro-resistant risedronate taken fasted or with food versus immediate-release risedronate.Pharmacol Res Perspect. 2022 Jun;10(3):e00957. doi: 10.1002/prp2.957. Pharmacol Res Perspect. 2022. PMID: 35526121 Free PMC article. Clinical Trial.
-
Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease.Drugs. 2001;61(5):685-712. doi: 10.2165/00003495-200161050-00013. Drugs. 2001. PMID: 11368289 Review.
-
Risedronate dosing before breakfast compared with dosing later in the day in women with postmenopausal osteoporosis.Osteoporos Int. 2009 Nov;20(11):1895-902. doi: 10.1007/s00198-009-0893-2. Epub 2009 Mar 19. Osteoporos Int. 2009. PMID: 19296144 Clinical Trial.
References
-
- Sietsema WK, Ebetino FH, Salvagno AM, Bevan JA. Antiresorptive dose–response relationships across three generations of bisphosphonates. Drugs Exp Clin Res. 1989;15:389–396. - PubMed
-
- Mortensen L, Charles P, Bekker PJ, DiGennaro J, Johnston CC. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998;83:396–402. - PubMed
-
- Hosking DJ, Eusebio RA, Chines AA. Paget’s disease of bone: reduction of disease activity with oral risedronate. Bone. 1998;22:51–55. - PubMed
-
- Siris ES, Chines AA, Altman RD, et al. Risedronate in the treatment of Paget’s disease of bone: an open label, multicenter study. J Bone Miner Res. 1998;13:1032–1038. - PubMed
-
- Singer FR, Clemens TL, Eusebio RA, Bekker PJ. Risedronate, a highly effective oral agent in the treatment of patients with severe Paget’s disease. J Clin Endocrinol Metab. 1998;83:1906–1910. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

