Nitric oxide and macrophage antiviral extrinsic activity
- PMID: 10583595
- PMCID: PMC2326934
- DOI: 10.1046/j.1365-2567.1999.00864.x
Nitric oxide and macrophage antiviral extrinsic activity
Abstract
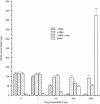
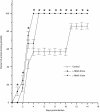
In this study we evaluated the relationship between nitric oxide (NO) and macrophage antiviral extrinsic activity. Macrophages activated by intraperitoneal injection of herpes simplex virus-2 (HSV-2), showed both extrinsic antiviral activity and high nitrite production in contrast to non-activated, resident macrophages. The extrinsic antiviral activity was observed in cultures of Vero cells infected with HSV-1 and HSV-2. The NO inhibitor N-monomethyl-l-arginine acetate (l-NMA) impaired the antiviral activity of HSV-elicited macrophages. The effect was dose dependent and correlated with a reduction of nitrite in the culture media. The effect of l-NMA was reversed by the addition of l-arginine. These data indicate that NO could be responsible for the described activity. Furthermore, l-NMA treatment resulted in the aggravation of HSV-1-induced keratitis in the mouse model, supporting a defensive role of NO in the pathogenesis of HSV-1 corneal infection.
Figures
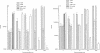
References
-
- Sit MF, Tenney DJ, Rothstein JL, Morahan PS. Effect of macrophage activation on resistance of mouse peritoneal macrophages to infection with herpes simplex virus types 1 and 2. J Gen Virol. 1988;69:1999. - PubMed
-
- Morahan PS, Morse S, McGeorge M. Macrophage extrinsic antiviral activity during herpes simplex virus infection. J Gen Virol. 1980;46:291. - PubMed
-
- Ellerman-Eriksen S. Autocrine secretion of interferon-α/β and tumour necrosis factor-α synergistically activates mouse macrophages after infection with herpes simplex virus type 2. J Gen Virol. 1993;74:2191. - PubMed
-
- Ellerman-Eriksen S, Sommerlund M, Mogensen SC. Differential sensitivity of macrophages from herpes simplex virus resistant and susceptible mice to respiratory burst priming by interferon-α/β. J Gen Virol. 1989;70:2139. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

