Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation
- PMID: 10583602
- PMCID: PMC2326945
- DOI: 10.1046/j.1365-2567.1999.00888.x
Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation
Abstract
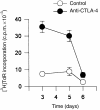
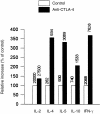
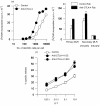
Occupancy of CTLA-4 (cytotoxic T-lymphocyte antigen-4 or CD152) negatively regulates the activation of mouse T lymphocytes, as indicated by the fate of CTLA-4-deficient mice, by the impact of anti-CTLA-4 monoclonal antibodies (mAbs) on mouse T-cell activation in vitro and by the impact of CTLA-4 blockade on the course of experimental tumoral, autoimmune, alloimmune or infectious disease in this animal. The function of human CTLA-4, however, remains less clear. The expression and function of human CTLA-4 were further explored. CTLA-4 was expressed under mitogenic conditions only, its expression being, at least partially, dependent on the secretion of interleukin-2. Memory T cells expressed CTLA-4 with faster kinetics than naive T cells. The functional role of human CTLA-4 was assessed utilizing a panel of four anti-CTLA-4 mAbs that blocked the interaction between CTLA-4 and its ligands. These mAbs, in immobilized form, profoundly inhibited the activation of T cells by immobilized anti-CD3 mAb in the absence of anti-CD28 mAb, but co-stimulated T-cell activation in the presence of anti-CD28 mAb. Finally, and importantly, blockade of the interaction of CTLA-4 with its ligands using soluble anti-CTLA-4 mAbs, in intact form or as Fab fragments, enhanced T-cell activation in several polyclonal or alloantigen-specific CD80- or CD80/CD86-dependent assays, as measured by cytokine production, cellular proliferation or cytotoxic responses. It is concluded that interaction of CTLA-4 with its functional ligands, CD80 or CD86, can down-regulate human T-cell responses, probably by intracellular signalling events and independent of CD28 occupancy.
Figures


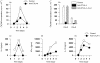
References
-
- June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321. - PubMed
-
- Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7 (CD80) and B7 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA‐4 receptors. Immunity. 1994;1:793. - PubMed
-
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA‐4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA‐4. Immunity. 1995;3:541. - PubMed
-
- Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla‐4. Science. 1995;270:985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

