Massive acinar cell apoptosis with secondary necrosis, origin of ducts in atrophic lobules and failure to regenerate in cyanohydroxybutene pancreatopathy in rats
- PMID: 10583631
- PMCID: PMC2517772
- DOI: 10.1046/j.1365-2613.1999.00117.x
Massive acinar cell apoptosis with secondary necrosis, origin of ducts in atrophic lobules and failure to regenerate in cyanohydroxybutene pancreatopathy in rats
Abstract
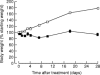
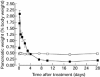
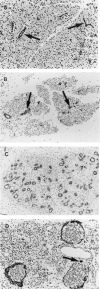
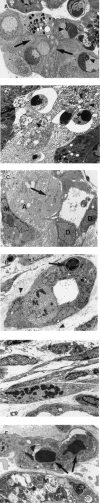
Cyanohydroxybutene (CHB), a glycosinolate breakdown product, causes pancreatic injury when given to animals in large amounts. To determine the course of CHB-induced pancreatopathy, rats were given a single subcutaneous dose of CHB and the pancreas weighed and examined by light and electron microscopy and immunohistochemistry at intervals from 2 h to 28 days. The pancreatic lesion was unusual in that there was marked early oedema with limited inflammatory cell infiltration, rapid synchronous onset of acinar cell apoptosis and early advanced atrophy engendering only a limited regenerative response. Acinar cell apoptosis was atypical in that cell fragmentation was limited and phagocytosis delayed, resulting in extensive secondary necrosis. As ducts were unaffected by CHB, the crowded ducts making up the epithelial component of atrophic lobules could be clearly shown to derive from their condensation and proliferation, not the redifferentiation of pre-existing acinar cells, widely held to produce this lesion. Although the basis of CHB selectivity and toxicity for pancreatic acinar cells remains unknown, the potential therapeutic benefit of such an agent in patients with pancreatitis or pancreatic tumours warrants further investigation.
Figures

Similar articles
-
Acinar cell apoptosis and the origin of tubular complexes in caerulein-induced pancreatitis.Int J Exp Pathol. 1999 Aug;80(4):205-15. doi: 10.1046/j.1365-2613.1999.00116.x. Int J Exp Pathol. 1999. PMID: 10583630 Free PMC article.
-
Pancreatic acinar cell regeneration following copper deficiency-induced pancreatic necrosis.Int J Pancreatol. 1987 Apr;2(2):71-85. doi: 10.1007/BF03015000. Int J Pancreatol. 1987. PMID: 3681036
-
Pancreatic necrosis and regeneration induced by 4-hydroxyaminoquinoline-1-oxide in the guinea pig.Lab Invest. 1975 Jan;32(1):98-104. Lab Invest. 1975. PMID: 1113507
-
What's new in in vitro studies of exocrine pancreatic cell injury?Pathol Res Pract. 1985 Mar;179(4-5):576-88. doi: 10.1016/S0344-0338(85)80199-0. Pathol Res Pract. 1985. PMID: 3889889 Review.
-
Morphology of the exocrine pancreas related to pancreatitis.Microsc Res Tech. 1997 Jun 1-15;37(5-6):509-19. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<509::AID-JEMT13>3.0.CO;2-U. Microsc Res Tech. 1997. PMID: 9220428 Review.
Cited by
-
Investigating the cause of Brassica-associated liver disease (BALD) in cattle: Progoitrin-derived nitrile toxicosis in rats.Toxicon X. 2019 Dec 30;5:100021. doi: 10.1016/j.toxcx.2019.100021. eCollection 2020 Mar. Toxicon X. 2019. PMID: 32550577 Free PMC article.
-
A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation.Exp Eye Res. 2007 May;84(5):894-904. doi: 10.1016/j.exer.2007.01.015. Epub 2007 Feb 4. Exp Eye Res. 2007. PMID: 17362931 Free PMC article.
-
Pharmacologically directed cell disposal: labeling damaged cells for phagocytosis as a strategy against acute pancreatitis.Mol Interv. 2010 Apr;10(2):80-5. doi: 10.1124/mi.10.2.9. Mol Interv. 2010. PMID: 20368368 Free PMC article. Review.
-
Acinar cell apoptosis and the origin of tubular complexes in caerulein-induced pancreatitis.Int J Exp Pathol. 1999 Aug;80(4):205-15. doi: 10.1046/j.1365-2613.1999.00116.x. Int J Exp Pathol. 1999. PMID: 10583630 Free PMC article.
-
Rapid hair cell loss: a mouse model for cochlear lesions.J Assoc Res Otolaryngol. 2008 Mar;9(1):44-64. doi: 10.1007/s10162-007-0105-8. Epub 2007 Dec 4. J Assoc Res Otolaryngol. 2008. PMID: 18057986 Free PMC article.
References
-
- Adler G, Hupp T, Kern HF. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch. [A] Path. Anat. Histol. 1979;382:31–47. - PubMed
-
- Ansari B, Coates PJ, Greenstein BD, Hall PA. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J. Pathol. 1993;170:1–8. - PubMed
-
- Barres BA, Hart IK, Coles HSR, Barne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
-
- Bell JM, Williams K. Growth-depressing factors in rapeseed oilmeal. Can. J. Agr. Sci. 1953;33:201–209.
-
- Bendayan M. Concentration of amylase along its secretory pathway in the pancreatic acinar cell as revealed by high resolution immunocytochemistry. Histochem. J. 1984;16:85–108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

