Secretion of cryparin, a fungal hydrophobin
- PMID: 10584000
- PMCID: PMC91740
- DOI: 10.1128/AEM.65.12.5431-5435.1999
Secretion of cryparin, a fungal hydrophobin
Abstract
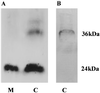
Cryparin is a cell-surface-associated hydrophobin of the filamentous ascomycete Cryphonectria parasitica. This protein contains a signal peptide that directs it to the vesicle-mediated secretory pathway. We detected a glycosylated form of cryparin in a secretory vesicle fraction, but secreted forms of this protein are not glycosylated. This glycosylation occurred in the proprotein region, which is cleaved during maturation by a Kex2-like serine protease, leaving a mature form of cryparin that could be isolated from both the cell wall and culture medium. Pulse-chase labeling experiments showed that cryparin was secreted through the cell wall, without being bound, into the culture medium. The secreted protein then binds to the cell walls of C. parasitica, where it remains. Binding of cryparin to the cell wall occurred in submerged culture, presumably because of the lectin-like properties unique to this hydrophobin. Thus, the binding of this hydrophobin to the cell wall is different from that of other hydrophobins which are reported to require a hydrophobic-hydrophilic interface for assembly.
Figures




Similar articles
-
The mycovirus CHV1 disrupts secretion of a developmentally regulated protein in Cryphonectria parasitica.J Virol. 2012 Jun;86(11):6067-74. doi: 10.1128/JVI.05756-11. Epub 2012 Mar 21. J Virol. 2012. PMID: 22438560 Free PMC article.
-
A Hydrophobin of the chestnut blight fungus, Cryphonectria parasitica, is required for stromal pustule eruption.Eukaryot Cell. 2005 May;4(5):931-6. doi: 10.1128/EC.4.5.931-936.2005. Eukaryot Cell. 2005. PMID: 15879527 Free PMC article.
-
Role of MAPK Signaling Pathways in Regulating the Hydrophobin Cryparin in the Chestnut Blight Fungus Cryphonectria parasitica.Mycobiology. 2017 Dec;45(4):362-369. doi: 10.5941/MYCO.2017.45.4.362. Epub 2017 Dec 31. Mycobiology. 2017. PMID: 29371804 Free PMC article.
-
Fungal Hydrophobins and Their Self-Assembly into Functional Nanomaterials.Adv Exp Med Biol. 2019;1174:161-185. doi: 10.1007/978-981-13-9791-2_5. Adv Exp Med Biol. 2019. PMID: 31713199 Review.
-
Hydrophobins, the fungal coat unravelled.Biochim Biophys Acta. 2000 Sep 18;1469(2):79-86. doi: 10.1016/s0304-4157(00)00002-2. Biochim Biophys Acta. 2000. PMID: 10998570 Review.
Cited by
-
Differential modulation of cellular signaling pathways by mild and severe hypovirus strains.Eukaryot Cell. 2002 Jun;1(3):401-13. doi: 10.1128/EC.1.3.401-413.2002. Eukaryot Cell. 2002. PMID: 12455988 Free PMC article.
-
A nutrient-regulated, dual localization phospholipase A(2) in the symbiotic fungus Tuber borchii.EMBO J. 2001 Sep 17;20(18):5079-90. doi: 10.1093/emboj/20.18.5079. EMBO J. 2001. PMID: 11566873 Free PMC article.
-
In Silico Evaluation, Phylogenetic Analysis, and Structural Modeling of the Class II Hydrophobin Family from Different Fungal Phytopathogens.Microorganisms. 2023 Oct 26;11(11):2632. doi: 10.3390/microorganisms11112632. Microorganisms. 2023. PMID: 38004644 Free PMC article.
-
Genome sequencing of Elaeocarpus spp. stem blight pathogen Pseudocryphonectria elaeocarpicola reveals potential adaptations to colonize woody bark.BMC Genomics. 2024 Jul 24;25(1):714. doi: 10.1186/s12864-024-10615-5. BMC Genomics. 2024. PMID: 39048950 Free PMC article.
-
Comparative Secretome Analysis Reveals Perturbation of Host Secretion Pathways by a Hypovirus.Sci Rep. 2016 Oct 4;6:34308. doi: 10.1038/srep34308. Sci Rep. 2016. PMID: 27698384 Free PMC article.
References
-
- Carpenter C E, Mueller R J, Kazmierczak P, Zhang L, Villalon D K, Van Alfen N K. Effect of a virus on accumulation of a tissue-specific cells-surface protein of the fungus Cryphonectria (Endothia) parasitica. Mol Plant-Microbe Interact. 1992;4:55–61. - PubMed
-
- Fahima T, Kazmierczak P, Hansen D R, Pfeiffer P, Van Alfen N K. Membrane-associated replication of an unencapsidated double-stranded RNA of the fungus Cryphonectria parasitica. Virology. 1993;195:81–89. - PubMed
-
- Hansen D R, Van Alfen N K, Gilles K, Powell W A. Naked dsRNA association with hypovirulence of Endothia parasitica is packaged in fungal vesicles. J Gen Virol. 1985;66:2605–2614.
-
- Julius D, Schekman R, Thorner J. Glycosylation and processing of prepro-α-factor through the yeast secretory pathway. Cell. 1984;36:309–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

