Initial reactions in anaerobic alkane degradation by a sulfate reducer, strain AK-01
- PMID: 10584014
- PMCID: PMC91754
- DOI: 10.1128/AEM.65.12.5532-5540.1999
Initial reactions in anaerobic alkane degradation by a sulfate reducer, strain AK-01
Abstract
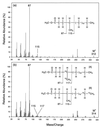
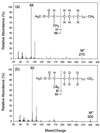
An alkane-degrading, sulfate-reducing bacterial strain, AK-01, isolated from a petroleum-contaminated sediment was studied to elucidate its mechanism of alkane metabolism. Total cellular fatty acids of AK-01 were predominantly C even when it was grown on C-even alkanes and were predominantly C odd when grown on C-odd alkanes, suggesting that the bacterium anaerobically oxidizes alkanes to fatty acids. Among these fatty acids, some 2-, 4-, and 6-methylated fatty acids were specifically found only when AK-01 was grown on alkanes, and their chain lengths always correlated with those of the alkanes. When [1,2-(13)C(2)]hexadecane or perdeuterated pentadecane was used as the growth substrate, (13)C-labeled 2-Me-16:0, 4-Me-18:0, and 6-Me-20:0 fatty acids or deuterated 2-Me-15:0, 4-Me-17:0, and 6-Me-19:0 fatty acids were recovered, respectively, confirming that these monomethylated fatty acids were alkane derived. Examination of the (13)C-labeled 2-, 4-, and 6-methylated fatty acids by mass spectrometry showed that each of them contained two (13)C atoms, located at the methyl group and the adjacent carbon, thus indicating that the methyl group was the original terminal carbon of the [1, 2-(13)C(2)]hexadecane. For perdeuterated pentadecane, the presence of three deuterium atoms, on the methyl group and its adjacent carbon, in each of the deuterated 2-, 4-, and 6-methylated fatty acids further supported the hypothesis that the methyl group was the terminal carbon of the alkane. Thus, exogenous carbon appears to be initially added to an alkane subterminally at the C-2 position such that the original terminal carbon of the alkane becomes a methyl group on the subsequently formed fatty acid. The carbon addition reaction, however, does not appear to be a direct carboxylation of inorganic bicarbonate. A pathway for anaerobic metabolism of alkanes by strain AK-01 is proposed.
Figures

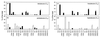



Similar articles
-
Anaerobic transformation of alkanes to fatty acids by a sulfate-reducing bacterium, strain Hxd3.Appl Environ Microbiol. 2003 Jul;69(7):3892-900. doi: 10.1128/AEM.69.7.3892-3900.2003. Appl Environ Microbiol. 2003. PMID: 12839758 Free PMC article.
-
Anaerobic n-alkane metabolism by a sulfate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803T.Appl Environ Microbiol. 2005 Jul;71(7):3458-67. doi: 10.1128/AEM.71.7.3458-3467.2005. Appl Environ Microbiol. 2005. PMID: 16000749 Free PMC article.
-
Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions.Arch Microbiol. 1998 Oct;170(5):361-9. doi: 10.1007/s002030050654. Arch Microbiol. 1998. PMID: 9818355
-
Metabolism of Hydrocarbons in n-Alkane-Utilizing Anaerobic Bacteria.J Mol Microbiol Biotechnol. 2016;26(1-3):138-51. doi: 10.1159/000442160. Epub 2016 Mar 10. J Mol Microbiol Biotechnol. 2016. PMID: 26959725 Review.
-
The anaerobic degradation of gaseous, nonmethane alkanes - From in situ processes to microorganisms.Comput Struct Biotechnol J. 2015 Mar 19;13:222-8. doi: 10.1016/j.csbj.2015.03.002. eCollection 2015. Comput Struct Biotechnol J. 2015. PMID: 25904994 Free PMC article. Review.
Cited by
-
Sulfate-Reducing Naphthalene Degraders Are Picky Eaters.Microorganisms. 2018 Jun 25;6(3):59. doi: 10.3390/microorganisms6030059. Microorganisms. 2018. PMID: 29941798 Free PMC article.
-
Effects of spilled oil on bacterial communities of mediterranean coastal anoxic sediments chronically subjected to oil hydrocarbon contamination.Microb Ecol. 2007 Nov;54(4):646-61. doi: 10.1007/s00248-007-9221-6. Epub 2007 Mar 4. Microb Ecol. 2007. PMID: 17334965
-
Recent advances in petroleum microbiology.Microbiol Mol Biol Rev. 2003 Dec;67(4):503-49. doi: 10.1128/MMBR.67.4.503-549.2003. Microbiol Mol Biol Rev. 2003. PMID: 14665675 Free PMC article. Review.
-
Anaerobic 1-alkene metabolism by the alkane- and alkene-degrading sulfate reducer Desulfatibacillum aliphaticivorans strain CV2803T.Appl Environ Microbiol. 2007 Dec;73(24):7882-90. doi: 10.1128/AEM.01097-07. Epub 2007 Oct 26. Appl Environ Microbiol. 2007. PMID: 17965214 Free PMC article.
-
Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture.Appl Environ Microbiol. 2000 Dec;66(12):5393-8. doi: 10.1128/AEM.66.12.5393-5398.2000. Appl Environ Microbiol. 2000. PMID: 11097919 Free PMC article.
References
-
- Aeckersberg F. Anaerober Abbau von Alkanen und 1-Alkenen durch sulfatreduzierende Bakterien. Ph.D. dissertation. Bremen, Germany: University of Bremen; 1994. . Verlag Mainz, Mainz, Germany.
-
- Aeckersberg F, Bak F, Widdel F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch Microbiol. 1991;156:5–14.
-
- Aeckersberg F, Rainey F A, Widdel F. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch Microbiol. 1998;170:361–369. - PubMed
-
- Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. - PubMed
-
- Blasig R, Huth J, Franke P, Borneleit P, Schunck W-H, Müller H-G. Degradation of long-chain n-alkanes by yeast Candida maltosa. III. Effect of solid n-alkanes on cellular fatty acid composition. Appl Microbiol Biotechnol. 1989;31:571–576.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

