Requirement for phosphoglucose isomerase of Xanthomonas campestris in pathogenesis of citrus canker
- PMID: 10584018
- PMCID: PMC91758
- DOI: 10.1128/AEM.65.12.5564-5573.1999
Requirement for phosphoglucose isomerase of Xanthomonas campestris in pathogenesis of citrus canker
Abstract
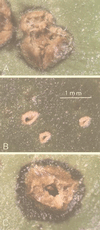
A mutant (XT906) of Xanthomonas campestris pv. citri, the causal agent of citrus canker, was induced by insertion of the transposon Tn5tac1 and isolated. This mutant did not grow or elicit canker disease in citrus leaves but was still able to induce a hypersensitive response in a nonhost plant (the common bean). The mutant was also unable to grow on minimal medium containing fructose or glycerol as the sole carbon source. A 2.5-kb fragment of wild-type DNA that complemented the mutant phenotype of XT906 was isolated. Sequence analysis revealed that this DNA fragment encoded a protein of 562 amino acids that shows homology to phosphoglucose isomerase (PGI). Enzyme activity assay confirmed that the encoded protein possesses PGI activity. Analysis of the activity of the promoter of the pgi gene revealed that it was inhibited by growth in complex medium but induced by culture in plant extract. These results demonstrate that PGI is required for pathogenicity of X. campestris pv. citri.
Figures
Similar articles
-
Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) Dye 1978 forms A, B/C/D, and E as X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov. nom. rev. comb. nov.; X. campestris pv malvacearum (ex smith 1901) Dye 1978 as X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) dye 1978 as X. alfalfae subsp. alfalfae (ex Riker et al., 1935) sp. nov. nom. rev.; and "var. fuscans" of X. campestris pv. phaseoli (ex Smith, 1987) Dye 1978 as X. fuscans subsp. fuscans sp. nov.Syst Appl Microbiol. 2005 Aug;28(6):494-518. doi: 10.1016/j.syapm.2005.03.017. Syst Appl Microbiol. 2005. PMID: 16104350
-
Genetic analysis of hrp-related DNA sequences of Xanthomonas campestris strains causing diseases of citrus.Appl Environ Microbiol. 1994 Apr;60(4):1078-86. doi: 10.1128/aem.60.4.1078-1086.1994. Appl Environ Microbiol. 1994. PMID: 7912499 Free PMC article.
-
An hrcU-homologous gene mutant of Xanthomonas campestris pv. glycines 8ra that lost pathogenicity on the host plant but was able to elicit the hypersensitive response on nonhosts.Mol Plant Microbe Interact. 1999 Jul;12(7):633-9. doi: 10.1094/MPMI.1999.12.7.633. Mol Plant Microbe Interact. 1999. PMID: 10478481
-
Requirement of the galU gene for polysaccharide production by and pathogenicity and growth In Planta of Xanthomonas citri subsp. citri.Appl Environ Microbiol. 2010 Apr;76(7):2234-42. doi: 10.1128/AEM.02897-09. Epub 2010 Jan 29. Appl Environ Microbiol. 2010. PMID: 20118360 Free PMC article.
-
Plant responses underlying nonhost resistance of Citrus limon against Xanthomonas campestris pv. campestris.Mol Plant Pathol. 2019 Feb;20(2):254-269. doi: 10.1111/mpp.12752. Epub 2018 Oct 31. Mol Plant Pathol. 2019. PMID: 30260546 Free PMC article.
Cited by
-
Expression profiling of virulence and pathogenicity genes of Xanthomonas axonopodis pv. citri.J Bacteriol. 2005 Feb;187(3):1201-5. doi: 10.1128/JB.187.3.1201-1205.2005. J Bacteriol. 2005. PMID: 15659697 Free PMC article.
-
Insights into Superinfection Immunity Regulation of Xanthomonas axonopodis Filamentous Bacteriophage cf.Curr Microbiol. 2023 Dec 19;81(1):42. doi: 10.1007/s00284-023-03539-y. Curr Microbiol. 2023. PMID: 38112972
-
Fructose-bisphophate aldolase exhibits functional roles between carbon metabolism and the hrp system in rice pathogen Xanthomonas oryzae pv. oryzicola.PLoS One. 2012;7(2):e31855. doi: 10.1371/journal.pone.0031855. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22384086 Free PMC article.
-
A putative glucose 6-phosphate isomerase has pleiotropic functions on virulence and other mechanisms in Acidovorax citrulli.Front Plant Sci. 2023 Nov 7;14:1275438. doi: 10.3389/fpls.2023.1275438. eCollection 2023. Front Plant Sci. 2023. PMID: 38023913 Free PMC article.
-
Comparative Omics Analysis of Historic and Recent Isolates of Bordetella pertussis and Effects of Genome Rearrangements on Evolution.Emerg Infect Dis. 2021 Jan;27(1):57-68. doi: 10.3201/eid2701.191541. Emerg Infect Dis. 2021. PMID: 33350934 Free PMC article.
References
-
- Ausubel F M, Brent R, Kingston R E, Moore S S, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 1. New York, N.Y: Wiley; 1987.
-
- Barber C E, Tang J L, Feng J X, Pan M Q, Wilson T J G, Slater H, Dow J M, Williams P, Daniels M J. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol. 1997;24:555–566. - PubMed
-
- Bonas U, Fenselau S, Horns T, Maric C, Moussian B, Pierre M, Wengelnik K, den Ackerveken V. hrpX and avirulence genes of Xanthomonas campestris pv. vesicatoria controlling the interaction with pepper and tomato. In: Daniels M J, et al., editors. Advances in molecular genetics of plant-microbe interactions. Vol. 3. Amsterdam, The Netherlands: Kluwer; 1994. pp. 57–64.
-
- Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;14:459–472. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

