Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi
- PMID: 10584019
- PMCID: PMC91759
- DOI: 10.1128/AEM.65.12.5571-5575.1999
Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi
Abstract
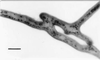
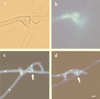
We observed anastomosis between hyphae originating from the same spore and from different spores of the same isolate of the arbuscular mycorrhizal fungi Glomus mosseae, Glomus caledonium, and Glomus intraradices. The percentage of contacts leading to anastomosis ranged from 35 to 69% in hyphae from the same germling and from 34 to 90% in hyphae from different germlings. The number of anastomoses ranged from 0.6 to 1.3 per cm (length) of hyphae in mycelia originating from the same spore. No anastomoses were observed between hyphae from the same or different germlings of Gigaspora rosea and Scutellospora castanea; no interspecific or intergeneric hyphal fusions were observed. We monitored anastomosis formation with time-lapse and video-enhanced light microscopy. We observed complete fusion of hyphal walls and the migration of a mass of particles in both directions within the hyphal bridges. In hyphal bridges of G. caledonium, light-opaque particles moved at the speed of 1.8 +/- 0.06 microm/s. We observed nuclear migration between hyphae of the same germling and between hyphae belonging to different germlings of the same isolate of three Glomus species. Our work suggests that genetic exchange may occur through intermingling of nuclei during anastomosis formation and opens the way to studies of vegetative compatibility in natural populations of arbuscular mycorrhizal fungi.
Figures


References
-
- Ainsworth A M, Rayner A D M. Responses of living hyphae associated with self and non-self fusions in the basidiomycete Phanerochaete velutina. J Gen Microbiol. 1986;132:191–201.
-
- Brasier C. A champion thallus. Nature. 1992;356:382–383.
-
- Burggraaf A J P, Beringer J E. Nuclear division and VA-mycorrhizal in vitro culture. In: Sylvia D M, Hung L L, Graham J H, editors. Mycorrhizae in the next decade. Gainesville: University of Florida; 1988. p. 190.
-
- Carlile M J. The success of the hypha and mycelium. In: Gow N A R, Gadd G M, editors. The growing fungus. London, United Kingdom: Chapman & Hall; 1995. pp. 3–19.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

