Pathology and epizootiology of Entomophaga maimaiga infections in forest Lepidoptera
- PMID: 10585966
- PMCID: PMC98977
- DOI: 10.1128/MMBR.63.4.814-835.1999
Pathology and epizootiology of Entomophaga maimaiga infections in forest Lepidoptera
Abstract
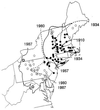
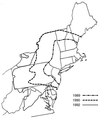
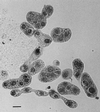
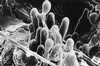
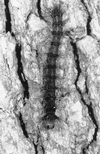
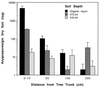
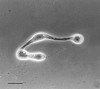
The insect-pathogenic fungal pathogen Entomophaga maimaiga is endemic to northeastern Asia and was first found in North America in 1989. Due to repeated epizootics and spread within populations of the major forest defoliator in northeastern North America, the gypsy moth (Lymantria dispar), this pathogen has gained much notoriety. Although this pathogen was purposely introduced to North America for biological control of L. dispar in 1910 to 1911, it is questionable whether it became established at the time of release and then remained at innocuous levels until relatively recently. Alternatively, the fungal strain present in North America today could be a more recent accidental introduction. DNA analysis demonstrates that this pathogen differs significantly from North American members of the same species complex (the Lepidoptera-specific Entomophaga aulicae species complex), and, to date, isolates of this introduced pathogen display little heterogeneity in North America. Nonsusceptible lepidopteran larvae have been identified, and either E. maimaiga is unable to penetrate the cuticle or the fungus cannot survive within the hemocoel. In the latter case, although E. maimaiga grows as protoplasts lacking cell walls in the host hemolymph, glycoproteins on plasma membranes of the protoplasts could lead to host recognition. Epizootiological studies demonstrate a clear association between fungal activity and environmental moisture but little association with host density under hypothesized conditions of high fungal density. Prediction of the occurrence of epizootics is not yet possible. E. maimaiga is easily established in new areas by releasing azygospores, but the ability to use this pathogen further for biological control will depend, in large part, on the development of mass production systems.
Figures
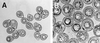


References
-
- Abrahamson L P, Harper J D. Microbial insecticides control forest tent caterpillar in southwestern Alabama. Forest Service research note SO-157. U.S. Washington, D.C.: Department of Agriculture; 1973.
-
- Aoki J. Mixed infection of the gypsy moth, Lymantria dispar japonica Motschulsky (Lepidoptera: Lymantriidae), in a larch forest by Entomophthora aulicae (Reich.) Sorok. and Paecilomyces canadensis (Vuill.) Brown et Smith. Appl Entomol Zool. 1974;9:185–190.
-
- Aoki J, Yanase K, Yanbe T, Koyama R. Hibernation of resting spores of Entomophthora aulicae in egg masses of the gypsy moth Porthetria dispar. J Invertebr Pathol. 1976;27:395–396.
-
- Aylor D E. A framework for examining inter-regional aerial transport of fungal spores. Agric For Meteorol. 1986;38:263–288.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

