Adding the third dimension to virus life cycles: three-dimensional reconstruction of icosahedral viruses from cryo-electron micrographs
- PMID: 10585969
- PMCID: PMC98980
- DOI: 10.1128/MMBR.63.4.862-922.1999
Adding the third dimension to virus life cycles: three-dimensional reconstruction of icosahedral viruses from cryo-electron micrographs
Erratum in
- Microbiol Mol Biol Rev 2000 Mar;64(1):237
Abstract
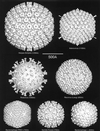
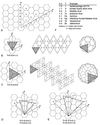
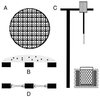
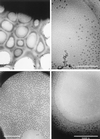
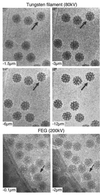
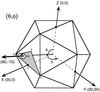
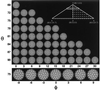
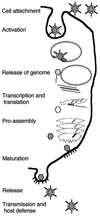
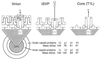
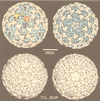
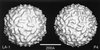
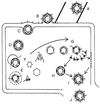
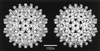
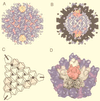
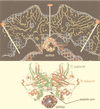
Viruses are cellular parasites. The linkage between viral and host functions makes the study of a viral life cycle an important key to cellular functions. A deeper understanding of many aspects of viral life cycles has emerged from coordinated molecular and structural studies carried out with a wide range of viral pathogens. Structural studies of viruses by means of cryo-electron microscopy and three-dimensional image reconstruction methods have grown explosively in the last decade. Here we review the use of cryo-electron microscopy for the determination of the structures of a number of icosahedral viruses. These studies span more than 20 virus families. Representative examples illustrate the use of moderate- to low-resolution (7- to 35-A) structural analyses to illuminate functional aspects of viral life cycles including host recognition, viral attachment, entry, genome release, viral transcription, translation, proassembly, maturation, release, and transmission, as well as mechanisms of host defense. The success of cryo-electron microscopy in combination with three-dimensional image reconstruction for icosahedral viruses provides a firm foundation for future explorations of more-complex viral pathogens, including the vast number that are nonspherical or nonsymmetrical.
Figures


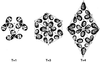







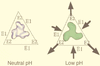

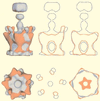


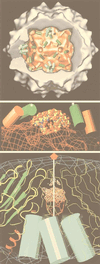


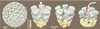
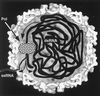
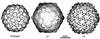
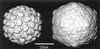











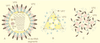



References
-
- Adrian M, Dubochet J, Lepault J, McDowall A W. Cryo-electron microscopy of viruses. Nature (London) 1984;308:32–36. - PubMed
-
- Aldroubi U, Trus B L, Unser M, Booy F P, Steven A C. Magnification mismatches between micrographs: corrective procedures and implications for structural analysis. Ultramicroscopy. 1992;46:175–188. - PubMed
-
- Anderer F A, Schlumberger H D, Koch M A, Frank H, Eggers H J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967;32:511–523. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

