Stress genes and proteins in the archaea
- PMID: 10585970
- PMCID: PMC98981
- DOI: 10.1128/MMBR.63.4.923-967.1999
Stress genes and proteins in the archaea
Abstract
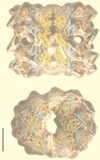
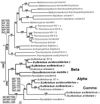
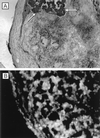
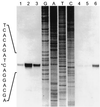
The field covered in this review is new; the first sequence of a gene encoding the molecular chaperone Hsp70 and the first description of a chaperonin in the archaea were reported in 1991. These findings boosted research in other areas beyond the archaea that were directly relevant to bacteria and eukaryotes, for example, stress gene regulation, the structure-function relationship of the chaperonin complex, protein-based molecular phylogeny of organisms and eukaryotic-cell organelles, molecular biology and biochemistry of life in extreme environments, and stress tolerance at the cellular and molecular levels. In the last 8 years, archaeal stress genes and proteins belonging to the families Hsp70, Hsp60 (chaperonins), Hsp40(DnaJ), and small heat-shock proteins (sHsp) have been studied. The hsp70(dnaK), hsp40(dnaJ), and grpE genes (the chaperone machine) have been sequenced in seven, four, and two species, respectively, but their expression has been examined in detail only in the mesophilic methanogen Methanosarcina mazei S-6. The proteins possess markers typical of bacterial homologs but none of the signatures distinctive of eukaryotes. In contrast, gene expression and transcription initiation signals and factors are of the eucaryal type, which suggests a hybrid archaeal-bacterial complexion for the Hsp70 system. Another remarkable feature is that several archaeal species in different phylogenetic branches do not have the gene hsp70(dnaK), an evolutionary puzzle that raises the important question of what replaces the product of this gene, Hsp70(DnaK), in protein biogenesis and refolding and for stress resistance. Although archaea are prokaryotes like bacteria, their Hsp60 (chaperonin) family is of type (group) II, similar to that of the eukaryotic cytosol; however, unlike the latter, which has several different members, the archaeal chaperonin system usually includes only two (in some species one and in others possibly three) related subunits of approximately 60 kDa. These form, in various combinations depending on the species, a large structure or chaperonin complex sometimes called the thermosome. This multimolecular assembly is similar to the bacterial chaperonin complex GroEL/S, but it is made of only the large, double-ring oligomers each with eight (or nine) subunits instead of seven as in the bacterial complex. Like Hsp70(DnaK), the archaeal chaperonin subunits are remarkable for their evolution, but for a different reason. Ubiquitous among archaea, the chaperonins show a pattern of recurrent gene duplication-hetero-oligomeric chaperonin complexes appear to have evolved several times independently. The stress response and stress tolerance in the archaea involve chaperones, chaperonins, other heat shock (stress) proteins including sHsp, thermoprotectants, the proteasome, as yet incompletely understood thermoresistant features of many molecules, and formation of multicellular structures. The latter structures include single- and mixed-species (bacterial-archaeal) types. Many questions remain unanswered, and the field offers extraordinary opportunities owing to the diversity, genetic makeup, and phylogenetic position of archaea and the variety of ecosystems they inhabit. Specific aspects that deserve investigation are elucidation of the mechanism of action of the chaperonin complex at different temperatures, identification of the partners and substitutes for the Hsp70 chaperone machine, analysis of protein folding and refolding in hyperthermophiles, and determination of the molecular mechanisms involved in stress gene regulation in archaeal species that thrive under widely different conditions (temperature, pH, osmolarity, and barometric pressure). These studies are now possible with uni- and multicellular archaeal models and are relevant to various areas of basic and applied research, including exploration and conquest of ecosystems inhospitable to humans and many mammals and plants.
Figures












Similar articles
-
The archaeal molecular chaperone machine: peculiarities and paradoxes.Genetics. 1999 Aug;152(4):1277-83. doi: 10.1093/genetics/152.4.1277. Genetics. 1999. PMID: 10430558 Free PMC article. Review.
-
Evolution of assisted protein folding: the distribution of the main chaperoning systems within the phylogenetic domain archaea.Front Biosci. 2004 May 1;9:1318-32. doi: 10.2741/1328. Front Biosci. 2004. PMID: 14977547 Review.
-
Molecular biology of stress genes in methanogens: potential for bioreactor technology.Adv Biochem Eng Biotechnol. 2003;81:95-150. doi: 10.1007/3-540-45839-5_4. Adv Biochem Eng Biotechnol. 2003. PMID: 12747562 Review.
-
The molecular chaperone system and other anti-stress mechanisms in archaea.Front Biosci. 2001 Feb 1;6:D262-83. doi: 10.2741/macario. Front Biosci. 2001. PMID: 11171552 Review.
-
The basal transcription factors TBP and TFB from the mesophilic archaeon Methanosarcina mazeii: structure and conformational changes upon interaction with stress-gene promoters.J Mol Biol. 2001 Jun 8;309(3):589-603. doi: 10.1006/jmbi.2001.4705. J Mol Biol. 2001. PMID: 11397082
Cited by
-
Comparative proteomic analysis of Methanothermobacter thermautotrophicus reveals methane formation from H2 and CO2 under different temperature conditions.Microbiologyopen. 2019 May;8(5):e00715. doi: 10.1002/mbo3.715. Epub 2018 Sep 10. Microbiologyopen. 2019. PMID: 30260585 Free PMC article.
-
Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana.Cell Stress Chaperones. 2001 Jul;6(3):201-8. doi: 10.1379/1466-1268(2001)006<0201:gaoths>2.0.co;2. Cell Stress Chaperones. 2001. PMID: 11599561 Free PMC article.
-
Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network.Microbiol Mol Biol Rev. 2002 Mar;66(1):64-93; table of contents. doi: 10.1128/MMBR.66.1.64-93.2002. Microbiol Mol Biol Rev. 2002. PMID: 11875128 Free PMC article. Review.
-
Intein Clustering Suggests Functional Importance in Different Domains of Life.Mol Biol Evol. 2016 Mar;33(3):783-99. doi: 10.1093/molbev/msv271. Epub 2015 Nov 25. Mol Biol Evol. 2016. PMID: 26609079 Free PMC article.
-
Biology and survival of extremely halophilic archaeon Haloarcula marismortui RR12 isolated from Mumbai salterns, India in response to salinity stress.Sci Rep. 2016 May 27;6:25642. doi: 10.1038/srep25642. Sci Rep. 2016. PMID: 27231230 Free PMC article.
References
-
- Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Pontén T, Alsmark U C M, Podowski R M, Näslund A K, Eriksson A-S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. - PubMed
-
- Andrä S, Frey G, Jaenicke R, Stetter K O. The thermosome from Methanopyrus kandleri possesses an NH4+-dependent ATPase activity. Eur J Biochem. 1998;255:93–99. - PubMed
-
- Andrä S, Frey G, Nitsch M, Baumeister W, Stetter K O. Purification and structural characterization of the thermosome from the hyperthermophilic archaeum Methanopyrus kandleri. FEBS Lett. 1996;379:127–131. - PubMed
-
- Aravind L, Tatusov R L, Wolf Y I, Walker D R, Koonin E V. Evidence for massive gene exchange between archaeal and bacterial hyperthermophiles. Trends Genet. 1998;14:442–444. - PubMed
-
- Archibald J M, Logsdon Jr J M, Doolittle W F. Recurrent paralogy in the evolution of archaeal chaperonins. Curr Biol. 1999;9:1053–1056. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

