A role for lipid rafts in B cell antigen receptor signaling and antigen targeting
- PMID: 10587346
- PMCID: PMC2195743
- DOI: 10.1084/jem.190.11.1549
A role for lipid rafts in B cell antigen receptor signaling and antigen targeting
Abstract
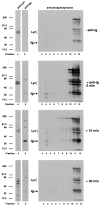
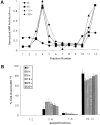
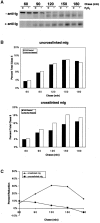
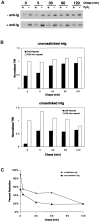
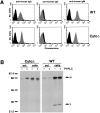
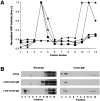
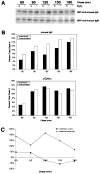
The B cell antigen receptor (BCR) serves both to initiate signal transduction cascades and to target antigen for processing and presentation by MHC class II molecules. How these two BCR functions are coordinated is not known. Recently, sphingolipid- and cholesterol-rich plasma membrane lipid microdomains, termed lipid rafts, have been identified and proposed to function as platforms for both receptor signaling and membrane trafficking. Here we show that upon cross-linking, the BCR rapidly translocates into ganglioside G(M1)-enriched lipid rafts that contain the Src family kinase Lyn and exclude the phosphatase CD45R. Both Igalpha and Lyn in the lipid rafts become phosphorylated, and subsequently the BCR and a portion of G(M1) are targeted to the class II peptide loading compartment. Entry into lipid rafts, however, is not sufficient for targeting to the antigen processing compartments, as a mutant surface Ig containing a deletion of the cytoplasmic domain is constitutively present in rafts but when cross-linked does not internalize to the antigen processing compartment. Taken together, these results provide evidence for a role for lipid rafts in the initial steps of BCR signaling and antigen targeting.
Figures



Similar articles
-
Floating the raft hypothesis for immune receptors: access to rafts controls receptor signaling and trafficking.Traffic. 2001 Mar;2(3):160-6. doi: 10.1034/j.1600-0854.2001.020302.x. Traffic. 2001. PMID: 11260521 Review.
-
Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR.Immunity. 2001 Jan;14(1):57-67. doi: 10.1016/s1074-7613(01)00089-9. Immunity. 2001. PMID: 11163230
-
B cell antigen receptor signaling links biochemical changes in the class II peptide-loading compartment to enhanced processing.Int Immunol. 1996 Dec;8(12):1867-76. doi: 10.1093/intimm/8.12.1867. Int Immunol. 1996. PMID: 8982771
-
Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling.J Immunol. 2001 Mar 15;166(6):3693-701. doi: 10.4049/jimmunol.166.6.3693. J Immunol. 2001. PMID: 11238609
-
Lipid rafts and B cell signaling.Semin Cell Dev Biol. 2007 Oct;18(5):616-26. doi: 10.1016/j.semcdb.2007.07.009. Epub 2007 Jul 24. Semin Cell Dev Biol. 2007. PMID: 17719248 Free PMC article. Review.
Cited by
-
Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation.Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3327-32. doi: 10.1073/pnas.0511315103. Epub 2006 Feb 21. Proc Natl Acad Sci U S A. 2006. PMID: 16492756 Free PMC article.
-
Visualization of negative signaling in B cells by quantitative confocal microscopy.Mol Cell Biol. 2001 Dec;21(24):8615-25. doi: 10.1128/MCB.21.24.8615-8625.2001. Mol Cell Biol. 2001. PMID: 11713294 Free PMC article.
-
Dynamics of membrane trafficking downstream of B and T cell receptor engagement: impact on immune synapses.Traffic. 2009 Jun;10(6):629-36. doi: 10.1111/j.1600-0854.2009.00913.x. Epub 2009 Mar 18. Traffic. 2009. PMID: 19416472 Free PMC article. Review.
-
Lipid rafts in immune signalling: current progress and future perspective.Immunology. 2016 Sep;149(1):13-24. doi: 10.1111/imm.12617. Epub 2016 Jul 11. Immunology. 2016. PMID: 27153983 Free PMC article. Review.
-
Ligand-independent signaling during early avian B cell development.Immunol Res. 2006;35(1-2):103-16. doi: 10.1385/IR:35:1:103. Immunol Res. 2006. PMID: 17003513 Review.
References
-
- Reth M., Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 1997;15:453–479. - PubMed
-
- Pleiman C.M., D'Ambrosia D., Cambier J.C. The B-cell antigen receptor complexstructure and signal transduction. Immunol. Today. 1994;15:393–399. - PubMed
-
- Gold M.R., DeFranco A.L. Biochemistry of B lymphocyte activation. Adv. Immunol. 1994;55:221–295. - PubMed
-
- Parker D.C. T cell-dependent B cell activation. Annu. Rev. Immunol. 1993;11:331–360. - PubMed
-
- Germain R.N. MHC-dependent antigen processing and peptide presentationproviding ligands for T lymphocyte activation. Cell. 1994;76:287–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

