Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis
- PMID: 10587515
- PMCID: PMC409856
- DOI: 10.1172/JCI6690
Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis
Abstract
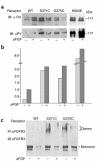
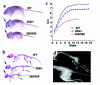
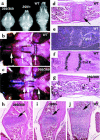
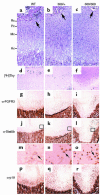
Missense mutations in fibroblast growth factor receptor 3 (FGFR3) result in several human skeletal dysplasias, including the most common form of dwarfism, achondroplasia. Here we show that a glycine-to-cysteine substitution at position 375 (Gly375Cys) in human FGFR3 causes ligand-independent dimerization and phosphorylation of FGFR3 and that the equivalent substitution at position 369 (Gly369Cys) in mouse FGFR3 causes dwarfism with features mimicking human achondroplasia. Accordingly, homozygous mice were more severely affected than heterozygotes. The resulting mutant mice exhibited macrocephaly and shortened limbs due to retarded endochondral bone growth and premature closure of cranial base synchondroses. Compared with their wild-type littermates, mutant mice growth plates shared an expanded resting zone and narrowed proliferating and hypertrophic zones, which is correlated with the activation of Stat proteins and upregulation of cell-cycle inhibitors. Reduced bone density is accompanied by increased activity of osteoclasts and upregulation of genes that are related to osteoblast differentiation, including osteopontin, osteonectin, and osteocalcin. These data reveal an essential role for FGF/FGFR3 signals in both chondrogenesis and osteogenesis during endochondral ossification.
Figures

Similar articles
-
A Lys644Glu substitution in fibroblast growth factor receptor 3 (FGFR3) causes dwarfism in mice by activation of STATs and ink4 cell cycle inhibitors.Hum Mol Genet. 1999 Jan;8(1):35-44. doi: 10.1093/hmg/8.1.35. Hum Mol Genet. 1999. PMID: 9887329
-
A Ser(365)-->Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia.Hum Mol Genet. 2001 Mar 1;10(5):457-65. doi: 10.1093/hmg/10.5.457. Hum Mol Genet. 2001. PMID: 11181569
-
Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype.Genes Dev. 2004 Feb 1;18(3):290-305. doi: 10.1101/gad.1179104. Genes Dev. 2004. PMID: 14871928 Free PMC article.
-
Mouse models orthologous to FGFR3-related skeletal dysplasias.Pediatr Pathol Mol Med. 2003 Jan-Feb;22(1):87-103. doi: 10.1080/pdp.22.1.87.103. Pediatr Pathol Mol Med. 2003. PMID: 12687892 Review.
-
Novel therapeutic approaches for the treatment of achondroplasia.Bone. 2020 Dec;141:115579. doi: 10.1016/j.bone.2020.115579. Epub 2020 Aug 11. Bone. 2020. PMID: 32795681 Review.
Cited by
-
Micro CT Analysis of Spine Architecture in a Mouse Model of Scoliosis.Front Endocrinol (Lausanne). 2015 Mar 19;6:38. doi: 10.3389/fendo.2015.00038. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25852647 Free PMC article.
-
Developmental Regulation of the Growth Plate and Cranial Synchondrosis.J Dent Res. 2016 Oct;95(11):1221-9. doi: 10.1177/0022034516651823. Epub 2016 Jun 1. J Dent Res. 2016. PMID: 27250655 Free PMC article. Review.
-
Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications.Cancer Metastasis Rev. 2015 Sep;34(3):479-96. doi: 10.1007/s10555-015-9579-8. Cancer Metastasis Rev. 2015. PMID: 26224133 Free PMC article. Review.
-
A novel FGFR3-binding peptide inhibits FGFR3 signaling and reverses the lethal phenotype of mice mimicking human thanatophoric dysplasia.Hum Mol Genet. 2012 Dec 15;21(26):5443-55. doi: 10.1093/hmg/dds390. Epub 2012 Sep 26. Hum Mol Genet. 2012. PMID: 23014564 Free PMC article.
-
N-cadherin and β1 integrin coordinately regulate growth plate cartilage architecture.Mol Biol Cell. 2024 Apr 1;35(4):ar49. doi: 10.1091/mbc.E23-03-0101. Epub 2024 Jan 31. Mol Biol Cell. 2024. PMID: 38294852 Free PMC article.
References
-
- Gilbert, S.F. 1994. The development of bones. In Developmental biology. 4th edition. S.F. Gilbert, editor. Sinauer Associates. Sunderland, MA. 333–338.
-
- Horton WA. Fibroblast growth factor receptor 3 and the human chondrodysplasias. Curr Opin Pediatr. 1997;9:437–442. - PubMed
-
- Muenke M, Schell U. Fibroblast-growth-factor receptor mutations in human skeletal disorders. Trends Genet. 1995;11:308–313. - PubMed
-
- Mulvihill JJ. Craniofacial syndromes: no such thing as a single gene disease. Nat Genet. 1995;9:101–103. - PubMed
-
- Rousseau F, et al. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

