Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis
- PMID: 10587518
- PMCID: PMC409862
- DOI: 10.1172/JCI7707
Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis
Abstract
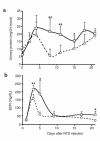
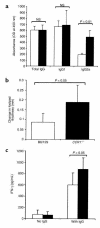
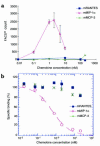
During the development of nephrotoxic nephritis (NTN) in the mouse, we find that a variety of chemokines and chemokine receptors are induced: CCR1 (RANTES, MIP-1alpha), CCR2 (MCP-1), CCR5 (RANTES, MIP-1alpha, MIP-1beta), CXCR2 (MIP-2), and CXCR3 (IP-10). Their timing of expression indicated that CXCR2 and CCR1 are probably important in the neutrophil-dependent heterologous phase of the disease, whereas CCR1, CCR2, CCR5, and CXCR3 accompany the subsequent mononuclear cell infiltration characteristic of autologous disease. We therefore assessed the role of CCR1 in NTN using CCR1(-/-) mice. We found that neutrophil accumulation in CCR1(-/-) mice was comparable to that in wild-type animals but that renal recruitment of CD4(+) and CD8(+) T cells and macrophages increased significantly. Moreover, CCR1(-/-) mice developed more severe glomerulonephritis than did controls, with greater proteinuria and blood urea nitrogen, as well as a higher frequency of crescent formation. In addition, CCR1(-/-) mice showed enhanced Th1 immune responses, including titers of antigen-specific IgG2a antibody, delayed-type hypersensitivity responses, and production of IFN-gamma and TNF-alpha. Lastly, using recombinant proteins and transfected cells that overexpressed CCR1, we demonstrated that MIP-1alpha, but not RANTES, bound CCR1 and induced cell chemotaxis. Thus, rather than simply promoting leukocyte recruitment during NTN, CCR1 expression profoundly alters the effector phase of glomerulonephritis. Therapeutic targeting of chemokine receptors may, on occasion, exacerbate underlying disease.
Figures




References
-
- Schreiner GF. The role of the macrophage in glomerular injury. Semin Nephrol. 1991;11:268–275. - PubMed
-
- Rovin BH, Phan LT. Chemotactic factors and renal inflammation. Am J Kidney Dis. 1998;31:1065–1084. - PubMed
-
- Rovin BH, Rumancik M, Tan L, Dickerson J. Glomerular expression of monocyte chemoattractant protein-1 in experimental and human glomerulonephritis. Lab Invest. 1994;71:536–542. - PubMed
-
- Tam FW, et al. Differential expression of macrophage inflammatory protein-2 and monocyte chemoattractant protein-1 in experimental glomerulonephritis. Kidney Int. 1996;49:715–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

