Hyposmolality stimulates apical membrane Na(+)/H(+) exchange and HCO(3)(-) absorption in renal thick ascending limb
- PMID: 10587523
- PMCID: PMC409859
- DOI: 10.1172/JCI7332
Hyposmolality stimulates apical membrane Na(+)/H(+) exchange and HCO(3)(-) absorption in renal thick ascending limb
Abstract
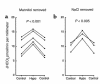
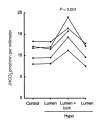
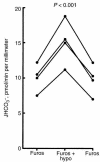
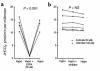
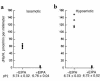
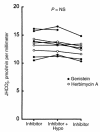
The regulation of epithelial Na(+)/H(+) exchangers (NHEs) by hyposmolality is poorly understood. In the renal medullary thick ascending limb (MTAL), transepithelial bicarbonate (HCO(3)(-)) absorption is mediated by apical membrane Na(+)/H(+) exchange, attributable to NHE3. In the present study we examined the effects of hyposmolality on apical Na(+)/H(+) exchange activity and HCO(3)(-) absorption in the MTAL of the rat. In MTAL perfused in vitro with 25 mM HCO(3)(-) solutions, decreasing osmolality in the lumen and bath by removal of either mannitol or sodium chloride significantly increased HCO(3)(-) absorption. The responses to lumen addition of the inhibitors ethylisopropyl amiloride, amiloride, or HOE 694 are consistent with hyposmotic stimulation of apical NHE3 activity and provide no evidence for a role for apical NHE2 in HCO(3)(-) absorption. Hyposmolality increased apical Na(+)/H(+) exchange activity over the pH(i) range 6.5-7.5 due to an increase in V(max). Pretreatment with either tyrosine kinase inhibitors or with the tyrosine phosphatase inhibitor molybdate completely blocked stimulation of HCO(3)(-) absorption by hyposmolality. These results demonstrate that hyposmolality increases HCO(3)(-) absorption in the MTAL through a novel stimulation of apical membrane Na(+)/H(+) exchange and provide the first evidence that NHE3 is regulated by hyposmotic stress. Stimulation of apical Na(+)/H(+) exchange activity in renal cells by a decrease in osmolality may contribute to such pathophysiological processes as urine acidification by diuretics, diuretic resistance, and renal sodium retention in edematous states.
Figures

Similar articles
-
Hyposmolality stimulates Na(+)/H(+) exchange and HCO(3)(-) absorption in thick ascending limb via PI 3-kinase.Am J Physiol Cell Physiol. 2000 Nov;279(5):C1443-54. doi: 10.1152/ajpcell.2000.279.5.C1443. Am J Physiol Cell Physiol. 2000. PMID: 11029292
-
Transepithelial HCO3- absorption is defective in renal thick ascending limbs from Na+/H+ exchanger NHE1 null mutant mice.Am J Physiol Renal Physiol. 2004 Dec;287(6):F1244-9. doi: 10.1152/ajprenal.00176.2004. Epub 2004 Aug 3. Am J Physiol Renal Physiol. 2004. PMID: 15292047
-
Hyperosmolality inhibits bicarbonate absorption in rat medullary thick ascending limb via a protein-tyrosine kinase-dependent pathway.J Biol Chem. 1995 Apr 28;270(17):9883-9. doi: 10.1074/jbc.270.17.9883. J Biol Chem. 1995. PMID: 7730371
-
H+ and HCO3- transporters in the medullary thick ascending limb of the kidney: molecular mechanisms, function and regulation.Kidney Int Suppl. 1998 Apr;65:S36-41. Kidney Int Suppl. 1998. PMID: 9551430 Review.
-
Bicarbonate transport along the loop of Henle: molecular mechanisms and regulation.J Nephrol. 2002 Mar-Apr;15 Suppl 5:S88-96. J Nephrol. 2002. PMID: 12027225 Review.
Cited by
-
Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes.Am J Physiol Renal Physiol. 2009 Oct;297(4):F866-74. doi: 10.1152/ajprenal.00335.2009. Epub 2009 Jul 22. Am J Physiol Renal Physiol. 2009. PMID: 19625374 Free PMC article.
-
A possible catalytic role for NH4+ in Na+ reabsorption across the thick ascending limb.Am J Physiol Renal Physiol. 2010 Mar;298(3):F510-1. doi: 10.1152/ajprenal.00678.2009. Epub 2009 Dec 9. Am J Physiol Renal Physiol. 2010. PMID: 20007343 Free PMC article. No abstract available.
-
Membrane surface charge dictates the structure and function of the epithelial Na+/H+ exchanger.EMBO J. 2011 Feb 16;30(4):679-91. doi: 10.1038/emboj.2010.356. Epub 2011 Jan 18. EMBO J. 2011. PMID: 21245831 Free PMC article.
-
Nerve growth factor inhibits Na+/H+ exchange and formula absorption through parallel phosphatidylinositol 3-kinase-mTOR and ERK pathways in thick ascending limb.J Biol Chem. 2008 Sep 26;283(39):26602-11. doi: 10.1074/jbc.M803019200. Epub 2008 Jul 25. J Biol Chem. 2008. PMID: 18660503 Free PMC article.
-
P2X receptors trigger intracellular alkalization in isolated perfused mouse medullary thick ascending limb.Acta Physiol (Oxf). 2015 Jan;213(1):277-84. doi: 10.1111/apha.12417. Epub 2014 Nov 24. Acta Physiol (Oxf). 2015. PMID: 25362991 Free PMC article.
References
-
- Grinstein S, Rotin D, Mason MJ. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989;988:73–97. - PubMed
-
- Noel J, Pouyssegur J. Hormonal regulation, pharmacology, and membrane sorting of verterbrate Na+/H+ exchanger isoforms. Am J Physiol. 1995;268:C283–C296. - PubMed
-
- Yun CHC, et al. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol. 1995;269:G1–G11. - PubMed
-
- Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev. 1997;77:51–74. - PubMed
-
- Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem. 1997;272:22373–22376. - PubMed

