Mechanical strain activates BNP gene transcription through a p38/NF-kappaB-dependent mechanism
- PMID: 10587524
- PMCID: PMC409860
- DOI: 10.1172/JCI7362
Mechanical strain activates BNP gene transcription through a p38/NF-kappaB-dependent mechanism
Abstract
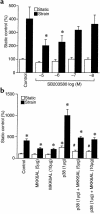
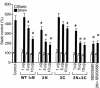
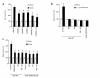
Application of mechanical strain to neonatal rat ventricular myocytes in culture evokes changes in gene expression reminiscent of those that occur with hypertrophy in vivo, such as stimulation of brain natriuretic peptide (BNP) gene expression. Here, we show that a major component of strain-dependent BNP promoter activation results from stimulation of p38 mitogen-activated protein kinase (MAPK) in the cardiac myocyte. Strain increased p38 activity in a time-dependent fashion. The p38 inhibitor SB203580 led to a reduction of approximately 60% in strain-activated human BNP (hBNP) promoter activity. Cotransfection of wild-type p38 increased both basal and strain-dependent promoter activity, while cotransfection with MKK6AL, a dominant-negative inhibitor of p38 MAPK kinase, resulted in partial inhibition of either p38- or strain-activated hBNP promoter activity. p38 MAPK increased hBNP promoter activity through activation of the transcription factor NF-kappaB. Activation of the hBNP promoter by either p38 or strain was mediated by DNA elements present in the 5' flanking sequence of the gene. Mechanical strain promoted assembly of NF-kappaB components on these DNA elements in vitro. Thus, induction of the hBNP promoter by mechanical strain depends, at least in part, on stimulation of p38 and subsequent activation of NF-kappaB. This activation may play an important role in signaling the increased BNP gene expression that accompanies hemodynamic overload and cardiac hypertrophy in vivo.
Figures


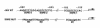
Similar articles
-
Endothelin-dependent and -independent components of strain-activated brain natriuretic peptide gene transcription require extracellular signal regulated kinase and p38 mitogen-activated protein kinase.Hypertension. 2000 Jan;35(1 Pt 2):188-92. doi: 10.1161/01.hyp.35.1.188. Hypertension. 2000. PMID: 10642296
-
Interleukin-1beta regulation of the human brain natriuretic peptide promoter involves Ras-, Rac-, and p38 kinase-dependent pathways in cardiac myocytes.Hypertension. 1999 Jan;33(1 Pt 2):283-9. doi: 10.1161/01.hyp.33.1.283. Hypertension. 1999. PMID: 9931118
-
Signaling mechanisms underlying strain-dependent brain natriuretic peptide gene transcription.Can J Physiol Pharmacol. 2001 Aug;79(8):640-5. Can J Physiol Pharmacol. 2001. PMID: 11558672
-
The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway.Br J Pharmacol. 2000 Sep;131(1):99-107. doi: 10.1038/sj.bjp.0703534. Br J Pharmacol. 2000. PMID: 10960075 Free PMC article.
-
Intracellular signaling in rat cultured vascular smooth muscle cells: roles of nuclear factor-kappaB and p38 mitogen-activated protein kinase on tumor necrosis factor-alpha production.Endocrinology. 1999 Aug;140(8):3562-72. doi: 10.1210/endo.140.8.6914. Endocrinology. 1999. PMID: 10433212
Cited by
-
Adenovirus 7 Induces Interlukin-6 Expression in Human Airway Epithelial Cells via p38/NF-κB Signaling Pathway.Front Immunol. 2020 Sep 23;11:551413. doi: 10.3389/fimmu.2020.551413. eCollection 2020. Front Immunol. 2020. PMID: 33072092 Free PMC article.
-
Signal transduction of mechanical stress in myocytes and fibroblasts derived from neonatal rat ventricles.Neth Heart J. 2001 Dec;9(9):372-378. Neth Heart J. 2001. PMID: 25696767 Free PMC article.
-
The relationship between serum NT-proBNP levels and severity of coronary artery disease assessed by SYNTAX score in patients with acute myocardial infarction.Turk J Med Sci. 2019 Oct 24;49(5):1366-1373. doi: 10.3906/sag-1902-26. Turk J Med Sci. 2019. PMID: 31648513 Free PMC article.
-
Natriuretic Peptides in the Regulation of Cardiovascular Physiology and Metabolic Events.J Am Heart Assoc. 2015 Oct 27;4(10):e002423. doi: 10.1161/JAHA.115.002423. J Am Heart Assoc. 2015. PMID: 26508744 Free PMC article. Review. No abstract available.
-
Knockout of Toll-Like Receptors 2 and 4 Prevents Renal Ischemia-Reperfusion-Induced Cardiac Hypertrophy in Mice.PLoS One. 2015 Oct 8;10(10):e0139350. doi: 10.1371/journal.pone.0139350. eCollection 2015. PLoS One. 2015. PMID: 26448184 Free PMC article.
References
-
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. - PubMed
-
- Komuro I, et al. Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem. 1990;265:3595–3598. - PubMed
-
- Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992;267:10551–10560. - PubMed
-
- Liang F, Wu J, Garami M, Gardner DG. Mechanical strain increases expression of the brain natriuretic peptide gene in rat cardiac myocytes. J Biol Chem. 1997;272:28050–28056. - PubMed
-
- Iwaki K, Sukhatme VP, Shubeita HE, Chien KR. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. J Biol Chem. 1990;265:13809–13817. - PubMed

