Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury
- PMID: 10587527
- PMCID: PMC409865
- DOI: 10.1172/JCI7903
Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury
Abstract
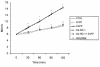
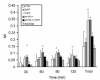
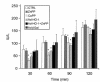
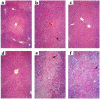
We examined the effects of upregulation of heme oxygenase-1 (HO-1) in steatotic rat liver models of ex vivo cold ischemia/reperfusion (I/R) injury. In the model of ischemia/isolated perfusion, treatment of genetically obese Zucker rats with the HO-1 inducer cobalt protoporphyrin (CoPP) or with adenoviral HO-1 (Ad-HO-1) significantly improved portal venous blood flow, increased bile production, and decreased hepatocyte injury. Unlike in untreated rats or those pretreated with the HO-1 inhibitor zinc protoporphyrin (ZnPP), upregulation of HO-1 by Western blots correlated with amelioration of histologic features of I/R injury. Adjunctive infusion of ZnPP abrogated the beneficial effects of Ad-HO-1 gene transfer, documenting the direct involvement of HO-1 in protection against I/R injury. Following cold ischemia/isotransplantation, HO-1 overexpression extended animal survival from 40% in untreated controls to about 80% after CoPP or Ad-HO-1 therapy. This effect correlated with preserved hepatic architecture, improved liver function, and depressed infiltration by T cells and macrophages. Hence, CoPP- or gene therapy-induced HO-1 prevented I/R injury in steatotic rat livers. These findings provide the rationale for refined new treatments that should increase the supply of usable donor livers and ultimately improve the overall success of liver transplantation.
Figures
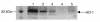



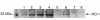
Comment in
-
Heme oxygenase-1: a redoubtable response that limits reperfusion injury in the transplanted adipose liver.J Clin Invest. 1999 Dec;104(11):1485-6. doi: 10.1172/JCI8827. J Clin Invest. 1999. PMID: 10587508 Free PMC article. No abstract available.
Similar articles
-
Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury.Transplantation. 2002 Jul 15;74(1):96-102. doi: 10.1097/00007890-200207150-00017. Transplantation. 2002. PMID: 12134106
-
Protective effect of heme oxygenase-1 induction against hepatic injury in alcoholic steatotic liver exposed to cold ischemia/reperfusion.Life Sci. 2012 Jan 30;90(5-6):169-76. doi: 10.1016/j.lfs.2011.10.003. Epub 2011 Oct 20. Life Sci. 2012. PMID: 22036622
-
Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation.Am J Transplant. 2001 Jul;1(2):121-8. Am J Transplant. 2001. PMID: 12099359
-
Heme Oxygenase-1 in liver transplant ischemia-reperfusion injury: From bench-to-bedside.Free Radic Biol Med. 2020 Sep;157:75-82. doi: 10.1016/j.freeradbiomed.2020.02.012. Epub 2020 Feb 19. Free Radic Biol Med. 2020. PMID: 32084514 Free PMC article. Review.
-
Protective role of heme oxygenase-1 in fatty liver ischemia-reperfusion injury.Med Mol Morphol. 2019 Jun;52(2):61-72. doi: 10.1007/s00795-018-0205-z. Epub 2018 Aug 31. Med Mol Morphol. 2019. PMID: 30171344 Free PMC article. Review.
Cited by
-
Therapeutic potential of heme oxygenase-1/carbon monoxide in lung disease.Int J Hypertens. 2012;2012:859235. doi: 10.1155/2012/859235. Epub 2012 Feb 1. Int J Hypertens. 2012. PMID: 22518295 Free PMC article.
-
Heme oxygenase-1, oxidation, inflammation, and atherosclerosis.Front Pharmacol. 2012 Jul 19;3:119. doi: 10.3389/fphar.2012.00119. eCollection 2012. Front Pharmacol. 2012. PMID: 22833723 Free PMC article.
-
Extraction of protoporphyrin disodium and its inhibitory effects on HBV-DNA.World J Gastroenterol. 2004 Feb 1;10(3):433-6. doi: 10.3748/wjg.v10.i3.433. World J Gastroenterol. 2004. PMID: 14760773 Free PMC article.
-
Upregulation of Carbonyl Reductase 1 by Nrf2 as a Potential Therapeutic Intervention for Ischemia/ Reperfusion Injury during Liver Transplantation.Mol Cells. 2019 Sep 30;42(9):672-685. doi: 10.14348/molcells.2019.0003. Mol Cells. 2019. PMID: 31486328 Free PMC article.
-
Heme oxygenase-1-dependent and -independent regulation of angiogenic genes expression: effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells.Cell Mol Biol (Noisy-le-grand). 2005 Sep 30;51(4):347-55. Cell Mol Biol (Noisy-le-grand). 2005. PMID: 16309584 Free PMC article.
References
-
- Markin RS, et al. Frozen section evaluation of donor livers before transplantation. Transplantation. 1993;56:1403–1409. - PubMed
-
- Hornboll P, Olsen TS. Fatty changes in the liver: the relation to age, overweight, and diabetes mellitus. Acta Pathol Microbiol Immunol Scand [A] 1982;90:199–205. - PubMed
-
- D’Allessandro AM, Kalayoglu M, Sollinger HW. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51:157–162. - PubMed
-
- Strasberg SM, Howard JK, Melmenti EP, Hersh M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. - PubMed
-
- Fellstrom B, Akuyrek LM, Zezina L. Post ischemic reperfusion injury and allograft arteriosclerosis. Transplant Proc. 1998;38:4278–4280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

