Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect
- PMID: 10587528
- PMCID: PMC483477
- DOI: 10.1172/JCI8390
Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect
Abstract
Several problems limit the application of gene transfer to correct the cystic fibrosis (CF) Cl(-) transport defect in airway epithelia. These include inefficient transduction with vectors applied to the apical surface, a low rate of division by airway epithelial cells, failure of transgene expression to persist, and immune responses to vectors or vector-encoded proteins. To address these issues, we used a feline immunodeficiency virus-based (FIV-based) vector. FIV vector formulated with a calcium chelator transduced fully differentiated, nondividing human airway epithelia when applied to the apical surface. FIV-based vector encoding the cystic fibrosis transmembrane conductance regulator cDNA corrected the Cl(-) transport defect in differentiated CF airway epithelia for the life of the culture (>3 months). When this approach was applied in vivo, FIV vector expressing beta-galactosidase transduced 1-14% of adult rabbit airway epithelia. Transduced cells were present in the conducting airways, bronchioles, and alveoli. Importantly, gene expression persisted, and cells with progenitor capacity were targeted. FIV-based lentiviral vectors may be useful for the treatment of genetic lung diseases such as CF. This article may have been published online in advance of the print edition.
Figures
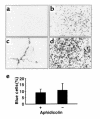
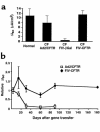

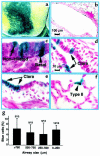
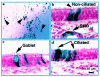
Comment in
-
Bad for cats, good for humans? Modified feline immunodeficiency virus for gene therapy.J Clin Invest. 1999 Dec;104(11):1491-3. doi: 10.1172/JCI8838. J Clin Invest. 1999. PMID: 10587510 Free PMC article. No abstract available.
References
-
- Welsh, M.J., Boat, T.F., Tsui, L.-C., and Beaudet, A.L. 1995. Cystic fibrosis. In The metabolic and molecular basis of inherited disease. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill Inc. New York, NY. 3799–3876.
-
- Ramsey BW. Drug therapy: management of pulmonary disease in patients with cystic fibrosis [review] N Engl J Med. 1996;335:179–188. - PubMed
-
- Rich DP, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347:358–363. - PubMed
-
- Drumm ML, et al. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990;62:1227–1233. - PubMed
-
- Miao CH, et al. The kinetics of rAAV integration in the liver. Nat Genet. 1998;19:13–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

