The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1
- PMID: 10588299
- PMCID: PMC1473211
- DOI: 10.1016/s0197-2456(99)00031-8
The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1
Abstract
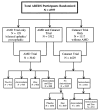
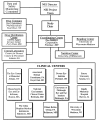
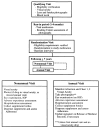
The Age-Related Eye Disease Study (AREDS) was initially conceived as a long-term multicenter, prospective study of the clinical course of age-related macular degeneration (AMD) and age-related cataract. Data on progression rates and risk factors from the study will increase understanding of the clinical course of both conditions, generate hypotheses about etiology, and aid in the design of clinical trials of potential interventions. In addition to collecting natural history data, AREDS includes a clinical trial of high-dose vitamin and mineral supplements for AMD and a clinical trial of high-dose vitamin supplements for cataract. The clinical trials were initiated largely because of the widespread public use in the United States of commercially available pharmacologic doses of vitamins and minerals to treat these two eye conditions and the absence of definitive studies on the safety and efficacy of their use. Important design issues for the clinical trials include: defining cataract and AMD, estimating event rates, determining the type and dosage of vitamins and minerals to be tested for each condition, and identifying the parameters necessary for monitoring safety and efficacy. This paper describes the AREDS design, including the study rationale and operational structure, and the approach adopted to combine, for two diseases, clinical trials with a natural history study.
Figures
References
-
- Sorsby A. Reports on Public Health and Medical Subjects. No 114. London: Her Majesty’s Stationary Office, 1966.
-
- Kahn HA, Moorhead HB. Statistics on Blindness in the Model Reporting Area. 1969–1970 Washington, D.C.: USDHEW, PHS Publication No. (NIH); 1973:73–427.
-
- National Advisory Eye Council. Report of the Retinal and Choroidal Diseases Panel. Vision Research-A National Plan: 1983–1987 Bethesda, MD: U.S. Department of Health and Human Services; 1984.
-
- National Advisory Eye Council. Report of the Cataract Panel. Vision Research—A National Plan: 1983–1987 Bethesda, MD: U.S. Department of Health and Human Services; 1984.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases