Degradation of a short-lived glycoprotein from the lumen of the endoplasmic reticulum: the role of N-linked glycans and the unfolded protein response
- PMID: 10588643
- PMCID: PMC25743
- DOI: 10.1091/mbc.10.12.4059
Degradation of a short-lived glycoprotein from the lumen of the endoplasmic reticulum: the role of N-linked glycans and the unfolded protein response
Abstract
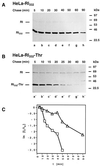
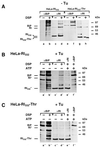
We are studying endoplasmic reticulum-associated degradation (ERAD) with the use of a truncated variant of the type I ER transmembrane glycoprotein ribophorin I (RI). The mutant protein, RI(332), containing only the N-terminal 332 amino acids of the luminal domain of RI, has been shown to interact with calnexin and to be a substrate for the ubiquitin-proteasome pathway. When RI(332) was expressed in HeLa cells, it was degraded with biphasic kinetics; an initial, slow phase of approximately 45 min was followed by a second phase of threefold accelerated degradation. On the other hand, the kinetics of degradation of a form of RI(332) in which the single used N-glycosylation consensus site had been removed (RI(332)-Thr) was monophasic and rapid, implying a role of the N-linked glycan in the first proteolytic phase. RI(332) degradation was enhanced when the binding of glycoproteins to calnexin was prevented. Moreover, the truncated glycoprotein interacted with calnexin preferentially during the first proteolytic phase, which strongly suggests that binding of RI(332) to the lectin-like protein may result in the slow, initial phase of degradation. Additionally, mannose trimming appears to be required for efficient proteolysis of RI(332). After treatment of cells with the inhibitor of N-glycosylation, tunicamycin, destruction of the truncated RI variants was severely inhibited; likewise, in cells preincubated with the calcium ionophore A23187, both RI(332) and RI(332)-Thr were stabilized, despite the presence or absence of the N-linked glycan. On the other hand, both drugs are known to trigger the unfolded protein response (UPR), resulting in the induction of BiP and other ER-resident proteins. Indeed, only in drug-treated cells could an interaction between BiP and RI(332) and RI(332)-Thr be detected. Induction of BiP was also evident after overexpression of murine Ire1, an ER transmembrane kinase known to play a central role in the UPR pathway; at the same time, stabilization of RI(332) was observed. Together, these results suggest that binding of the substrate proteins to UPR-induced chaperones affects their half lives.
Figures









References
-
- Brodsky JL, McCracken AA. ER-associated and proteasome-mediated protein degradation: how two topologically restricted events come together. Trends Cell Biol. 1997;7:151–156. - PubMed
-
- Chapman R, Sidrauski C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu Rev Cell Dev Biol. 1998;14:459–485. - PubMed
-
- Chen Y, Caherec F, Chuck SL. Calnexin and other factors that alter translocation affect the rapid binding of ubiquitin to apoB in the Sec61 complex. J Biol Chem. 1998;273:11887–11894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

