Two components of actin-based retrograde flow in sea urchin coelomocytes
- PMID: 10588644
- PMCID: PMC25744
- DOI: 10.1091/mbc.10.12.4075
Two components of actin-based retrograde flow in sea urchin coelomocytes
Abstract
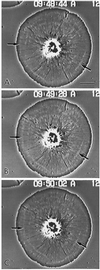
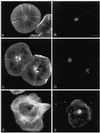
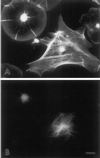
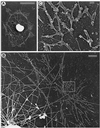
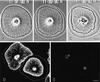
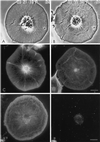
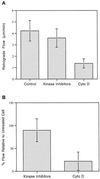
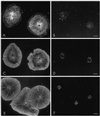
Sea urchin coelomocytes represent an excellent experimental model system for studying retrograde flow. Their extreme flatness allows for excellent microscopic visualization. Their discoid shape provides a radially symmetric geometry, which simplifies analysis of the flow pattern. Finally, the nonmotile nature of the cells allows for the retrograde flow to be analyzed in the absence of cell translocation. In this study we have begun an analysis of the retrograde flow mechanism by characterizing its kinetic and structural properties. The supramolecular organization of actin and myosin II was investigated using light and electron microscopic methods. Light microscopic immunolocalization was performed with anti-actin and anti-sea urchin egg myosin II antibodies, whereas transmission electron microscopy was performed on platinum replicas of critical point-dried and rotary-shadowed cytoskeletons. Coelomocytes contain a dense cortical actin network, which feeds into an extensive array of radial bundles in the interior. These actin bundles terminate in a perinuclear region, which contains a ring of myosin II bipolar minifilaments. Retrograde flow was arrested either by interfering with actin polymerization or by inhibiting myosin II function, but the pathway by which the flow was blocked was different for the two kinds of inhibitory treatments. Inhibition of actin polymerization with cytochalasin D caused the actin cytoskeleton to separate from the cell margin and undergo a finite retrograde retraction. In contrast, inhibition of myosin II function either with the wide-spectrum protein kinase inhibitor staurosporine or the myosin light chain kinase-specific inhibitor KT5926 stopped flow in the cell center, whereas normal retrograde flow continued at the cell periphery. These differential results suggest that the mechanism of retrograde flow has two, spatially segregated components. We propose a "push-pull" mechanism in which actin polymerization drives flow at the cell periphery, whereas myosin II provides the tension on the actin cytoskeleton necessary for flow in the cell interior.
Figures



Similar articles
-
Wound closure in the lamellipodia of single cells: mediation by actin polymerization in the absence of an actomyosin purse string.Mol Biol Cell. 2002 Mar;13(3):1001-14. doi: 10.1091/mbc.01-04-0167. Mol Biol Cell. 2002. PMID: 11907278 Free PMC article.
-
Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II.J Cell Sci. 2000 Feb;113 ( Pt 3):471-82. doi: 10.1242/jcs.113.3.471. J Cell Sci. 2000. PMID: 10639334
-
Decreased phosphorylation of four 20-kDa proteins precedes staurosporine-induced disruption of the actin/myosin cytoskeleton in rat astrocytes.Exp Cell Res. 1994 Sep;214(1):55-66. doi: 10.1006/excr.1994.1233. Exp Cell Res. 1994. PMID: 8082748
-
Myosins, Actin and Autophagy.Traffic. 2016 Aug;17(8):878-90. doi: 10.1111/tra.12410. Epub 2016 May 31. Traffic. 2016. PMID: 27146966 Free PMC article. Review.
-
Force propagation across cells: mechanical coherence of dynamic cytoskeletons.Curr Opin Cell Biol. 2009 Feb;21(1):47-50. doi: 10.1016/j.ceb.2009.01.020. Epub 2009 Feb 7. Curr Opin Cell Biol. 2009. PMID: 19208463 Free PMC article. Review.
Cited by
-
Biomechanical Aspects of Actin Bundle Dynamics.Front Cell Dev Biol. 2020 Jun 9;8:422. doi: 10.3389/fcell.2020.00422. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32582705 Free PMC article.
-
Sea urchin Paracentrotus lividus immune cells in culture: formulation of the appropriate harvesting and culture media and maintenance conditions.Biol Open. 2019 Mar 5;8(3):bio039289. doi: 10.1242/bio.039289. Biol Open. 2019. PMID: 30718227 Free PMC article.
-
Recombinant SpTransformer proteins bind to specific sites on sea urchin phagocytes and modulate SpTransformer gene expression and immune responsiveness.Front Immunol. 2025 Jan 28;15:1496832. doi: 10.3389/fimmu.2024.1496832. eCollection 2024. Front Immunol. 2025. PMID: 39936151 Free PMC article.
-
Coelomocyte populations in the sea urchin, Strongylocentrotus purpuratus, undergo dynamic changes in response to immune challenge.Front Immunol. 2022 Aug 31;13:940852. doi: 10.3389/fimmu.2022.940852. eCollection 2022. Front Immunol. 2022. PMID: 36119116 Free PMC article.
-
Extending the molecular clutch beyond actin-based cell motility.New J Phys. 2014 Oct;16(10):105012. doi: 10.1088/1367-2630/16/10/105012. New J Phys. 2014. PMID: 25383039
References
-
- Bray D, White JG. Cortical flow in animal cells. Science. 1988;239:883–888. - PubMed
-
- Bryan J, Kane RE. Actin gelation in sea urchin egg extracts. Methods Cell Biol. 1982;25:175–199. - PubMed
-
- Cheney RE, Riley MA, Mooseker MS. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993;24:215–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

