The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity
- PMID: 10588647
- PMCID: PMC25747
- DOI: 10.1091/mbc.10.12.4121
The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity
Abstract
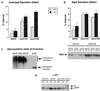
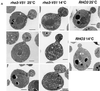
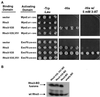
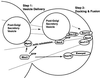
Budding yeast grow asymmetrically by the polarized delivery of proteins and lipids to specific sites on the plasma membrane. This requires the coordinated polarization of the actin cytoskeleton and the secretory apparatus. We identified Rho3 on the basis of its genetic interactions with several late-acting secretory genes. Mutational analysis of the Rho3 effector domain reveals three distinct functions in cell polarity: regulation of actin polarity, transport of exocytic vesicles from the mother cell to the bud, and docking and fusion of vesicles with the plasma membrane. We provide evidence that the vesicle delivery function of Rho3 is mediated by the unconventional myosin Myo2 and that the docking and fusion function is mediated by the exocyst component Exo70. These data suggest that Rho3 acts as a key regulator of cell polarity and exocytosis, coordinating several distinct events for delivery of proteins to specific sites on the cell surface.
Figures





References
-
- Aspenström P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. - PubMed
-
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. - PubMed
-
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. - PubMed
-
- Chant J, Pringle JR. Budding and cell polarity in Saccharomyces cerevisiae. Curr Opin Genet Dev. 1991;3:342–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

