Identification of an early T cell progenitor for a pathway of T cell maturation in the bone marrow
- PMID: 10588733
- PMCID: PMC24464
- DOI: 10.1073/pnas.96.25.14493
Identification of an early T cell progenitor for a pathway of T cell maturation in the bone marrow
Abstract
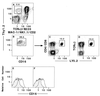
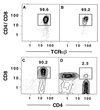
We have identified a rare ( approximately 0.05-0.1%) population of cells (Thy-1(hi)CD16(+)CD44(hi)CD2(-)TCRalphabeta(-)B220(-)M ac-1(-)NK1. 1(-)) in the adult mouse bone marrow that generates CD4(+) and CD8(+) TCRalphabeta(+) T cells after tissue culture for 48 hr in the presence of Ly5 congenic marrow cells. The essential stages in the maturation of the progenitors were determined; the stages included an early transition from CD2(-)CD16(+)CD44(hi)TCRalphabeta(-) to CD2(+)CD16(int/-)CD44(int/-)TCRalphabeta(-) cells, and a later transition to CD4(+)CD8(+)TCRalphabeta(+) double-positive T cells that rapidly generate the CD4(+) and CD8(+) single-positive T cells. The maturation of the progenitors is almost completely arrested at the CD2(+)TCRalphabeta(-) stage by the presence of mature T cells at the initiation of cultures. This alternate pathway is supported by the marrow microenvironment; it recapitulates critical intermediary steps in intrathymic T cell maturation.
Figures

Similar articles
-
T lymphocyte differentiation in vitro from adult human prethymic CD34+ bone marrow cells.J Exp Med. 1993 Jun 1;177(6):1531-9. doi: 10.1084/jem.177.6.1531. J Exp Med. 1993. PMID: 7684429 Free PMC article.
-
Scheduled kinetics of cell proliferation and phenotypic changes during immature thymocyte generation.Eur J Immunol. 2001 Oct;31(10):3038-47. doi: 10.1002/1521-4141(2001010)31:10<3038::aid-immu3038>3.0.co;2-3. Eur J Immunol. 2001. PMID: 11592080
-
p38 MAP kinase activity modulates alpha beta T cell development.Eur J Immunol. 2001 Oct;31(10):3056-63. doi: 10.1002/1521-4141(2001010)31:10<3056::aid-immu3056>3.0.co;2-b. Eur J Immunol. 2001. PMID: 11592082
-
Double-negative (CD4-CD8-), TCR alpha beta-expressing, peripheral T cells.Scand J Immunol. 1991 Dec;34(6):679-88. doi: 10.1111/j.1365-3083.1991.tb01592.x. Scand J Immunol. 1991. PMID: 1836273 Review. No abstract available.
-
TCRαβ+CD3+CD4-CD8- (double negative) T cells in autoimmunity.Autoimmun Rev. 2018 Apr;17(4):422-430. doi: 10.1016/j.autrev.2018.02.001. Epub 2018 Feb 9. Autoimmun Rev. 2018. PMID: 29428806 Review.
Cited by
-
T lymphoid differentiation in human bone marrow.Proc Natl Acad Sci U S A. 2003 May 27;100(11):6747-52. doi: 10.1073/pnas.1031503100. Epub 2003 May 8. Proc Natl Acad Sci U S A. 2003. PMID: 12738882 Free PMC article.
-
Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis.Blood. 2009 Jan 22;113(4):807-15. doi: 10.1182/blood-2008-08-173682. Epub 2008 Oct 16. Blood. 2009. PMID: 18927436 Free PMC article.
-
Signal integration and crosstalk during thymocyte migration and emigration.Nat Rev Immunol. 2011 Jun 24;11(7):469-77. doi: 10.1038/nri2989. Nat Rev Immunol. 2011. PMID: 21701522 Free PMC article. Review.
-
Differences in Bcl-2 expression by T-cell subsets alter their balance after in vivo irradiation to favor CD4+Bcl-2hi NKT cells.Eur J Immunol. 2009 Mar;39(3):763-75. doi: 10.1002/eji.200838657. Eur J Immunol. 2009. PMID: 19197937 Free PMC article.
-
Clonable progenitors committed to the T lymphocyte lineage in the mouse bone marrow; use of an extrathymic pathway.Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7455-60. doi: 10.1073/pnas.131559798. Epub 2001 Jun 5. Proc Natl Acad Sci U S A. 2001. PMID: 11390986 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

