Prevention of lymphocyte cell death in sepsis improves survival in mice
- PMID: 10588741
- PMCID: PMC24472
- DOI: 10.1073/pnas.96.25.14541
Prevention of lymphocyte cell death in sepsis improves survival in mice
Abstract
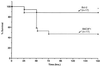
Sepsis induces extensive lymphocyte apoptosis, a process which may be beneficial to host survival by down-regulating the inflammatory response or, alternatively, harmful by impairing host defenses. To determine the beneficial vs. adverse effects of lymphocyte apoptosis in sepsis, we blocked lymphocyte apoptosis either by N-benzyloxycarbonyl-Val-Ala-Asp(O-methyl) fluoromethyl ketone (z-VAD), a broad-spectrum caspase inhibitor, or by use of Bcl-2 Ig transgenic mice that selectively overexpress the antiapoptotic protein Bcl-2 in a lymphoid pattern. Both z-VAD and Bcl-2 prevented lymphocyte apoptosis and resulted in a marked improvement in survival. z-VAD did not decrease lymphocyte tumor necrosis factor-alpha production. Considered together, these two studies employing different methods of blocking lymphocyte apoptosis provide compelling evidence that immunodepression resulting from the loss of lymphocytes is a central pathogenic event in sepsis, and they challenge the current paradigm that regards sepsis as a disorder resulting from an uncontrolled inflammatory response. Caspase inhibitors may represent a treatment strategy in this highly lethal disorder.
Figures




References
-
- Stone R. Science. 1994;264:365–367. - PubMed
-
- Wang S D, Huang K J, Lin Y S, Lei H Y. J Immunol. 1994;152:5014–5021. - PubMed
-
- Ayala A, Herndon C, Lehman D, DeMaso C, Ayala C, Chaudry I. Shock. 1995;3:259–268. - PubMed
-
- Hotchkiss R S, Swanson P E, Cobb J P, Jacobson A, Buchman T G, Karl I. Crit Care Med. 1997;25:1298–1307. - PubMed
-
- Bone R C. Crit Care Med. 1996;24:1125–1129. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

