Permeant ion regulation of N-methyl-D-aspartate receptor channel block by Mg(2+)
- PMID: 10588746
- PMCID: PMC24477
- DOI: 10.1073/pnas.96.25.14571
Permeant ion regulation of N-methyl-D-aspartate receptor channel block by Mg(2+)
Abstract
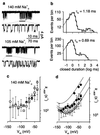
Block of the channel of N-methyl-D-aspartate (NMDA) receptors by external Mg(2+) (Mg(o)(2+)) has broad implications for the many physiological and pathological processes that depend on NMDA receptor activation. An essential property of channel block by Mg(o)(2+) is its powerful voltage dependence. A widely cited explanation for the strength of the voltage dependence of block is that the Mg(o)(2+)-binding site is located deep in the channel of NMDA receptors; Mg(o)(2+) then would sense most of the membrane potential field during block. However, recent electrophysiological and mutagenesis studies suggest that the blocking site cannot be deep enough to account for the voltage dependence of Mg(o)(2+) block. Here we describe the basis for this discrepancy: the magnitude and voltage dependence of channel block by Mg(o)(2+) are strongly regulated by external and internal permeant monovalent cations. Our data support a model in which access to the channel by Mg(o)(2+) is prevented when permeant ion-binding sites at the external entrance to the channel are occupied. Mg(o)(2+) can block the channel only when the permeant ion-binding sites are unoccupied and then can either unblock back to the external solution or permeate the channel. Unblock to the external solution is prevented if external permeant ions bind while Mg(2+) blocks the channel, although permeation is still permitted. The model provides an explanation for the strength of the voltage dependence of Mg(o)(2+) block and quantifies the interdependence of permanent and blocking ion binding to NMDA receptors.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

