Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection
- PMID: 10590100
- PMCID: PMC111522
Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection
Abstract
Antigens derived from host cells are detectable in the envelope of human immunodeficiency virus type 1 (HIV-1) and result in a distinctive viral phenotype reflecting that of the host cell. An immunomagnetic capture assay targeting discriminatory host proteins was developed to differentiate between HIV-1 derived from macrophages and lymphocytes. HIV-1 propagated in macrophages or lymphocytes in vitro was selectively captured by monoclonal antibodies directed against the virally incorporated cell-type-specific host markers CD36 (macrophages) and CD26 (lymphocytes). Furthermore, by targeting these markers, virus of defined cellular origin was selectively captured from a mixed pool of in vitro-propagated viruses. This technique was further refined in order to determine the impact of opportunistic infection on HIV-1 expression from these cellular compartments in vivo. Analysis of cell-free virus purified from plasma of patients with HIV-1 infection suggested that in those with an opportunistic infection, viral replication occurred in activated lymphocytes. Interestingly, there was also significant replication in activated macrophages in those patients with untreated pulmonary tuberculosis. Thus, in addition to lymphocytes, the macrophage cellular pool may serve as an important source of cell-free HIV-1 in patients with opportunistic infections that lead to marked macrophage activation. This novel viral capture technique may allow researchers to address a wide range of important questions regarding virus-host dynamics.
Figures

 ) was captured selectively by use of anti-CD3, anti-CD25, and anti-CD26. Antibodies to antigens common to both T lymphocytes and macrophages (HLA-A/B/C, HLA-DR, and CD44) captured both viral stocks. Data are representative of three independent experiments with <20% variability in the magnitude of capture.
) was captured selectively by use of anti-CD3, anti-CD25, and anti-CD26. Antibodies to antigens common to both T lymphocytes and macrophages (HLA-A/B/C, HLA-DR, and CD44) captured both viral stocks. Data are representative of three independent experiments with <20% variability in the magnitude of capture.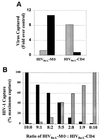
 ) and not by anti-CD36, indicating a discriminating phenotype for identifying the cellular origin of viral replication. (B) HIV-1Ba-L-MΦ and HIV-1Ba-L-CD4 isolates were mixed at various ratios and then captured with both anti-CD36 (■) and anti-CD26 (
) and not by anti-CD36, indicating a discriminating phenotype for identifying the cellular origin of viral replication. (B) HIV-1Ba-L-MΦ and HIV-1Ba-L-CD4 isolates were mixed at various ratios and then captured with both anti-CD36 (■) and anti-CD26 ( ). The amount of virus captured by each antibody was proportional to the input of each type of virus, further illustrating the selective capture of virus derived from diverse cell types. Data are representative of three independent experiments.
). The amount of virus captured by each antibody was proportional to the input of each type of virus, further illustrating the selective capture of virus derived from diverse cell types. Data are representative of three independent experiments.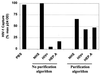
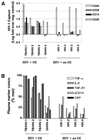
References
-
- Arthur L O, Bess J W, Jr, Sowder R C, Benveniste R E, Mann D L, Chermann J C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implication for pathogenesis and vaccines. Science. 1992;258:1935–1938. - PubMed
-
- Bourinbaiar A S. The ratio of defective HIV-1 particles to replication-competent infectious virions. Acta Virol. 1994;38:59–61. - PubMed
-
- Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. - PubMed
-
- Dierich M P, Frank I, Stoiber A, Clivio M, Spruth M, Katinger H W. The envelope of HIV. Immunol Lett. 1996;54:205–206. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
