RNA dimerization defect in a Rous sarcoma virus matrix mutant
- PMID: 10590103
- PMCID: PMC111525
- DOI: 10.1128/jvi.74.1.164-172.2000
RNA dimerization defect in a Rous sarcoma virus matrix mutant
Abstract
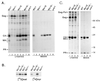
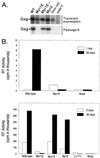
The retrovirus matrix (MA) sequence of the Gag polyprotein has been shown to contain functions required for membrane targeting and binding during particle assembly and budding. Additional functions for MA have been proposed based on the existence of MA mutants in Rous sarcoma virus (RSV), murine leukemia virus, human immunodeficiency virus type 1, and human T-cell leukemia virus type 1 that lack infectivity even though they release particles of normal composition. Here we describe an RSV MA mutant with a surprising and previously unreported phenotype. In the mutant known as Myr1E, the small membrane-binding domain of the Src oncoprotein has been added as an N-terminal extension of Gag. While Myr1E is not infectious, full infectivity can be reestablished by a single amino acid substitution in the Src sequence (G2E), which eliminates the addition of myristic acid and the membrane-binding capacity of this foreign sequence. The presence of myristic acid at the N terminus of the Myr1E Gag protein does not explain its replication defect, because other myristylated derivatives of RSV Gag are fully infectious (e.g., Myr2 [C. R. Erdie and J. W. Wills, J. Virol. 64:5204-5208, 1990]). Biochemical analyses of Myr1E particles reveal that they contain wild-type levels of the Gag cleavage products, Env glycoproteins, and reverse transcriptase activity when measured on an exogenous template. Genomic RNA incorporation appears to be mildly reduced compared to the wild-type level. Unexpectedly, RNA isolated from Myr1E particles is monomeric when analyzed on nondenaturing Northern blots. Importantly, the insertional mutation does not lie within previously identified dimer linkage sites. In spite of the dimerization defect, the genomic RNA from Myr1E particles serves efficiently as a template for reverse transcription as measured by an endogenous reverse transcriptase assay. In marked contrast, after infection of avian cells, the products of reverse transcription are nearly undetectable. These findings might be explained either by the loss of a normal function of MA needed in the formation or stabilization of RNA dimers or by the interference in such events by the mutant MA molecules. It is possible that Myr1E viruses package a single copy of viral RNA.
Figures





References
-
- Berkowitz R, Fisher J, Goff S P. RNA packaging. In: Krausslich H-G, editor. Morphogenesis and maturation of retroviruses. Berlin, Germany: Springer-Verlag; 1996. pp. 177–218.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

