Foot-and-mouth disease virus 3C protease induces cleavage of translation initiation factors eIF4A and eIF4G within infected cells
- PMID: 10590115
- PMCID: PMC111537
- DOI: 10.1128/jvi.74.1.272-280.2000
Foot-and-mouth disease virus 3C protease induces cleavage of translation initiation factors eIF4A and eIF4G within infected cells
Abstract
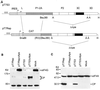
Infection of cells by foot-and-mouth disease virus (FMDV) results in the rapid inhibition of host cell protein synthesis. This process is accompanied by the early cleavage of the translation initiation factor eIF4G, a component of the cap-binding complex eIF4F. This cleavage is mediated by the leader (L) protease. Subsequently, as the virus proteins accumulate, secondary cleavages of eIF4G occur. Furthermore, eIF4A (46 kDa), a second component of eIF4F, is also cleaved in these later stages of the infection cycle. The 33-kDa cleavage product of eIF4A has lost a fragment from its N terminus. Transient-expression assays demonstrated that eIF4A was not cleaved in the presence of FMDV L or with the poliovirus 2A protease (which also mediates eIF4G cleavage) but was cleaved when the FMDV 3C protease was expressed. The FMDV 3C protease was also shown in such assays to induce cleavage of eIF4G, resulting in the production of cleavage products different from those generated by the L protease. Consistent with these results, within cells infected with a mutant FMDV lacking the L protease or within cells containing an FMDV replicon lacking L-P1 coding sequences it was again shown that eIF4A and eIF4G were cleaved.
Figures





References
-
- Belsham G J, Abrams C A, King A M Q, Roosien J, Vlak J M. Myristoylation of foot-and-mouth disease virus capsid protein precursors is independent of other viral proteins and occurs in both mammalian and insect cells. J Gen Virol. 1991;72:747–751. - PubMed
-
- Belsham G J. Analysis of picornavirus internal ribosome entry site function in vivo. In: Richter J, editor. mRNA formation and function. New York, N.Y: Academic Press; 1997. pp. 323–340.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

