A cysteine-rich motif in poliovirus protein 2C(ATPase) is involved in RNA replication and binds zinc in vitro
- PMID: 10590122
- PMCID: PMC111544
- DOI: 10.1128/jvi.74.1.334-343.2000
A cysteine-rich motif in poliovirus protein 2C(ATPase) is involved in RNA replication and binds zinc in vitro
Abstract
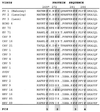
Protein 2C(ATPase) of picornaviruses is involved in the rearrangement of host cell organelles, viral RNA replication, and encapsidation. However, the biochemical and molecular mechanisms by which 2C(ATPase) engages in these processes are not known. To characterize functional domains of 2C(ATPase), we have focused on a cysteine-rich motif near the carboxy terminus of poliovirus 2C(ATPase). This region, which is well conserved among enteroviruses and rhinoviruses displaying an amino acid arrangement resembling zinc finger motifs, was studied by genetic and biochemical analyses. A mutation that replaced the first cysteine residue of the motif with a serine was lethal. A mutant virus which lacked the second of four potential coordination sites for zinc was temperature sensitive. At the restrictive temperature, RNA replication was inhibited whereas translation and polyprotein processing, assayed in vitro and in vivo, appeared to be normal. An intragenomic second-site revertant which reinserted the missing coordination site for zinc and recovered RNA replication at the restrictive temperature was isolated. The cysteine-rich motif was sufficient to bind zinc in vitro, as assessed in the presence of 4-(2-pyridylazo)resorcinol by a colorimetric assay. Zinc binding, however, was not required for hydrolysis of ATP. 2C(ATPase) as well as its precursors 2BC and P2 were found to exist in a reduced form in poliovirus-infected cells.
Figures





Similar articles
-
A C-terminal, cysteine-rich site in poliovirus 2C(ATPase) is required for morphogenesis.J Gen Virol. 2014 Jun;95(Pt 6):1255-1265. doi: 10.1099/vir.0.062497-0. Epub 2014 Feb 20. J Gen Virol. 2014. PMID: 24558221 Free PMC article.
-
A Single Amino Acid Substitution in Poliovirus Nonstructural Protein 2CATPase Causes Conditional Defects in Encapsidation and Uncoating.J Virol. 2016 Jun 24;90(14):6174-6186. doi: 10.1128/JVI.02877-15. Print 2016 Jul 15. J Virol. 2016. PMID: 27076638 Free PMC article.
-
Alanine scanning of poliovirus 2CATPase reveals new genetic evidence that capsid protein/2CATPase interactions are essential for morphogenesis.J Virol. 2012 Sep;86(18):9964-75. doi: 10.1128/JVI.00914-12. Epub 2012 Jul 3. J Virol. 2012. PMID: 22761387 Free PMC article.
-
Poliovirus protein 2C has ATPase and GTPase activities.J Biol Chem. 1993 Apr 15;268(11):8105-10. J Biol Chem. 1993. PMID: 8385138
-
Picornavirus 2C proteins: structure-function relationships and interactions with host factors.Front Cell Infect Microbiol. 2024 Feb 23;14:1347615. doi: 10.3389/fcimb.2024.1347615. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38465233 Free PMC article. Review.
Cited by
-
Conversion of VPg into VPgpUpUOH before and during poliovirus negative-strand RNA synthesis.J Virol. 2009 Dec;83(24):12660-70. doi: 10.1128/JVI.01676-08. Epub 2009 Oct 7. J Virol. 2009. PMID: 19812161 Free PMC article.
-
Poliovirus 2C protein forms homo-oligomeric structures required for ATPase activity.J Biol Chem. 2009 Aug 14;284(33):22012-22021. doi: 10.1074/jbc.M109.031807. Epub 2009 Jun 11. J Biol Chem. 2009. PMID: 19520852 Free PMC article.
-
HLA Class II molecules on haplotypes associated with type 1 diabetes exhibit similar patterns of binding affinities for coxsackievirus P2C peptides.Immunology. 2005 Nov;116(3):337-46. doi: 10.1111/j.1365-2567.2005.02233.x. Immunology. 2005. PMID: 16236123 Free PMC article.
-
Stimulation of poliovirus RNA synthesis and virus maturation in a HeLa cell-free in vitro translation-RNA replication system by viral protein 3CDpro.Virol J. 2005 Nov 21;2:86. doi: 10.1186/1743-422X-2-86. Virol J. 2005. PMID: 16300678 Free PMC article.
-
The identification and characterization of nucleic acid chaperone activity of human enterovirus 71 nonstructural protein 3AB.Virology. 2014 Sep;464-465:353-364. doi: 10.1016/j.virol.2014.07.037. Epub 2014 Aug 9. Virology. 2014. PMID: 25113906 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

