Polyuridylated mRNA synthesized by a recombinant influenza virus is defective in nuclear export
- PMID: 10590131
- PMCID: PMC111553
- DOI: 10.1128/jvi.74.1.418-427.2000
Polyuridylated mRNA synthesized by a recombinant influenza virus is defective in nuclear export
Abstract
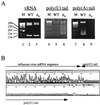
The poly(A) tail of influenza virus mRNA is synthesized by reiterative copying of a U track near the 5' end of the virion RNA (vRNA) template by the viral RNA polymerase. We have engineered a novel influenza A/WSN/33 virus which contains a neuraminidase (NA) vRNA with its U track mutated into an A track. Instead of synthesizing poly(A)-tailed NA mRNA, this novel virus synthesizes poly(U)-tailed NA mRNA. In infected cells, most poly(U)-tailed NA mRNA was retained in the nucleus, while most control polyadenylated NA mRNA was transported to the cytoplasm. These results suggest that the poly(A) tail is important for efficient nuclear export of NA mRNA. The mutant virus produced a reduced amount of NA and showed an attenuated phenotype, suggesting that poly(A) signal mutants of this type might be useful as potential live attenuated virus vaccines. In addition, this virus mutant might provide a useful model to further elucidate the basic mechanisms of mRNA nuclear export.
Figures





Similar articles
-
Direct evidence that the poly(A) tail of influenza A virus mRNA is synthesized by reiterative copying of a U track in the virion RNA template.J Virol. 1999 Apr;73(4):3473-6. doi: 10.1128/JVI.73.4.3473-3476.1999. J Virol. 1999. PMID: 10074205 Free PMC article.
-
Attenuation of influenza A virus mRNA levels by promoter mutations.J Virol. 1998 Aug;72(8):6283-90. doi: 10.1128/JVI.72.8.6283-6290.1998. J Virol. 1998. PMID: 9658066 Free PMC article.
-
The position 4 nucleotide at the 3' end of the influenza virus neuraminidase vRNA is involved in temporal regulation of transcription and replication of neuraminidase RNAs and affects the repertoire of influenza virus surface antigens.J Gen Virol. 1998 Aug;79 ( Pt 8):1923-34. doi: 10.1099/0022-1317-79-8-1923. J Gen Virol. 1998. PMID: 9714240
-
Transcription and replication of the influenza a virus genome.Acta Virol. 2000 Oct;44(5):273-82. Acta Virol. 2000. PMID: 11252672 Review.
-
Nuclear functions of the influenza A and B viruses NS1 proteins: do they play a role in viral mRNA export?Vaccine. 2009 Oct 23;27(45):6312-6. doi: 10.1016/j.vaccine.2009.01.015. Vaccine. 2009. PMID: 19840666 Review.
Cited by
-
Mutagenesis studies suggest a mechanism for influenza polymerase stalling during polyadenylation.Nucleic Acids Res. 2025 Jan 24;53(3):gkae1225. doi: 10.1093/nar/gkae1225. Nucleic Acids Res. 2025. PMID: 39676676 Free PMC article.
-
Messenger RNAs that are not synthesized by RNA polymerase II can be 3' end cleaved and polyadenylated.EMBO Rep. 2000 Dec;1(6):513-8. doi: 10.1093/embo-reports/kvd111. EMBO Rep. 2000. PMID: 11263496 Free PMC article.
-
Regulation of coronaviral poly(A) tail length during infection is not coronavirus species- or host cell-specific.Virus Genes. 2014 Dec;49(3):383-92. doi: 10.1007/s11262-014-1103-7. Epub 2014 Jul 18. Virus Genes. 2014. PMID: 25034371 Free PMC article.
-
The splicing factor proline-glutamine rich (SFPQ/PSF) is involved in influenza virus transcription.PLoS Pathog. 2011 Nov;7(11):e1002397. doi: 10.1371/journal.ppat.1002397. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22114566 Free PMC article.
-
Genetic analysis of influenza virus NS1 gene: a temperature-sensitive mutant shows defective formation of virus particles.J Virol. 2005 Dec;79(24):15246-57. doi: 10.1128/JVI.79.24.15246-15257.2005. J Virol. 2005. PMID: 16306596 Free PMC article.
References
-
- Alonso-Caplen F V, Nemeroff M E, Qiu Y, Krug R M. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 1992;6:255–267. - PubMed
-
- Colgan D F, Manley J L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766. - PubMed
-
- Dreyfuss G, Matunis M J, Piñol-Roma S, Burd C G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

