Brain infection by neuroinvasive but avirulent murine oncornaviruses
- PMID: 10590136
- PMCID: PMC111558
- DOI: 10.1128/jvi.74.1.465-473.2000
Brain infection by neuroinvasive but avirulent murine oncornaviruses
Abstract
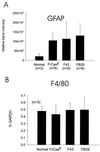
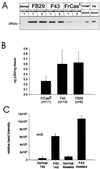
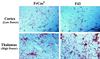
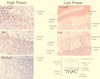
The chimeric murine oncornavirus FrCas(E) causes a rapidly progressive noninflammatory spongiform encephalomyelopathy after neonatal inoculation. The virus was constructed by the introduction of pol-env sequences from the wild mouse virus CasBrE into the genome of a neuroinvasive but nonneurovirulent strain of Friend murine leukemia virus (FMuLV), FB29. Although the brain infection by FrCas(E) as well as that by other neurovirulent murine retroviruses has been described in detail, little attention has been paid to the neuroinvasive but nonneurovirulent viruses. The purpose of the present study was to compare brain infection by FrCas(E) with that by FB29 and another nonneurovirulent virus, F43, which contains pol-env sequences from FMuLV 57. Both FB29 and F43 infected the same spectrum of cell types in the brain as that infected by FrCas(E), including endothelial cells, microglia, and populations of neurons which divide postnatally. Viral burdens achieved by the two nonneurovirulent viruses in the brain were actually higher than that of FrCas(E). The widespread infection of microglia by the two nonneurovirulent viruses is notable because it is infection of these cells by FrCas(E) which is thought to be a critical determinant of its neuropathogenicity. These results indicate that although the sequence of the envelope gene determines neurovirulence, this effect appears to operate through a mechanism which does not influence either viral tropism or viral burden in the brain. Although all three viruses exhibited similar tropism for granule neurons in the cerebellar cortex, there was a striking difference in the distribution of envelope proteins in those cells in vivo. The FrCas(E) envelope protein accumulated in terminal axons, whereas those of FB29 and F43 remained predominantly in the cell bodies. These observations suggest that differences in the intracellular sorting of these proteins may exist and that these differences appear to correlate with neurovirulence.
Figures



References
-
- Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. - PubMed
-
- Andersson P B, Perry V H, Gordon S. The kinetics and morphological characteristics of the macrophage-microglial response to kainic acid-induced neuronal degeneration. Neuroscience. 1991;42:201–214. - PubMed
-
- Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

