Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum
- PMID: 10590151
- PMCID: PMC111573
- DOI: 10.1128/jvi.74.1.564-572.2000
Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum
Abstract
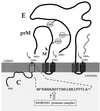
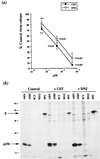
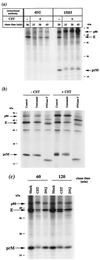
We report that endoplasmic reticulum alpha-glucosidase inhibitors have antiviral effects on dengue (DEN) virus. We found that glucosidase inhibition strongly affects productive folding pathways of the envelope glycoproteins prM (the intracellular glycosylated precursor of M [membrane protein]) and E (envelope protein): the proper folding of prM bearing unprocessed N-linked oligosaccharide is inefficient, and this causes delayed formation of prME heterodimer. The complexes formed between incompletely folded prM and E appear to be unstable, leading to a nonproductive pathway. Inhibition of alpha-glucosidase-mediated N-linked oligosaccharide trimming may thus prevent the assembly of DEN virus by affecting the early stages of envelope glycoprotein processing.
Figures

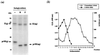


References
-
- Block T M, Lu X, Mehta A, Blumberg B S, Tennant B, Ebling M, Korba B, Lansky D M, Jacob G S, Dwek R A. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat Med. 1998;4:610–614. - PubMed
-
- Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

