Hypericum extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks
- PMID: 10591711
- PMCID: PMC28296
- DOI: 10.1136/bmj.319.7224.1534
Hypericum extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks
Erratum in
- BMJ 2000 Feb 5;320(7231):361
Abstract
Objectives: To assess the efficacy and safety of hypericum extract (STEI 300, Steiner Arzneimittel, Berlin) compared with imipramine and placebo in patients in primary care with a current episode of moderate depression.
Design: Randomised, double blind, multicentre, parallel group trial for 8 weeks.
Setting: Trained panel of 18 general practitioners from four German states: Bavaria, Berlin, Rhineland Palatinate, and Saxony.
Participants: 263 patients (66 men, 197 women) with moderate depression according to ICD-10 (international classification of diseases, 10th revision) codes F32. 1 and F33.1.
Interventions: 1050 mg hypericum extract (350 mg three times daily), 100 mg imipramine (50 mg, 25 mg, and 25 mg daily), or placebo three times daily.
Main outcome measures: Change from baseline score on the 17 item version of the Hamilton depression scale, the Hamilton anxiety scale, the clinical global impressions scale, Zung's self rating depression scale, and SF-36, and adverse events profile.
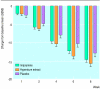
Results: Hypericum extract was more effective at reducing Hamilton depression scores than placebo and as effective as imipramine (mean -15.4 (SD 8.1), -12.1 (7.4), and -14.2 (7.3) respectively). Comparable results were found for Hamilton anxiety and clinical global impressions scales and were most pronounced for the Zung self rating depression scale. Quality of life was more improved in the standardised mental component scale of the SF-36 with both active treatments than with placebo but in the physical component scale was improved only by hypericum extract compared with placebo. The rate of adverse events with hypericum extract was in the range of the placebo group but lower than that of the imipramine group (0.5, 0.6, and 1.2 events per patient respectively).
Conclusions: At an average dose of 350 mg three times daily hypericum extract was more effective than placebo and at least as effective as 100 mg imipramine daily in the treatment of moderate depression. Treatment with hypericum extract is safe and improves quality of life.
Figures

Comment in
-
Commentary: has hypericum found its place in antidepressant treatment?BMJ. 1999 Dec 11;319(7224):1539. BMJ. 1999. PMID: 10651472 No abstract available.
-
Use of hypericum as antidepressant. Valid measure of antidepressant efficacy in primary care is needed.BMJ. 2000 Apr 22;320(7242):1141; author reply 1143. BMJ. 2000. PMID: 10775231 Free PMC article. No abstract available.
-
Use of hypericum as antidepressant. Naturalistic studies are needed.BMJ. 2000 Apr 22;320(7242):1141-2; author reply 1143. BMJ. 2000. PMID: 10836813 No abstract available.
-
Use of hypericum as antidepressant. Active substances must be identified.BMJ. 2000 Apr 22;320(7242):1142; author reply 1143. BMJ. 2000. PMID: 10836814 No abstract available.
-
Use of hypericum as antidepressant. The herbalist will see you now.BMJ. 2000 Apr 22;320(7242):1142; author reply 1143. BMJ. 2000. PMID: 10836815 No abstract available.
-
Use of hypericum as antidepressant. Use of placebo in depression trial was unethical.BMJ. 2000 Apr 22;320(7242):1142; author reply 1143. BMJ. 2000. PMID: 10836816 Clinical Trial. No abstract available.
-
Use of hypericum as antidepressant. Safety in overdose needs to be established.BMJ. 2000 Apr 22;320(7242):1142-3. BMJ. 2000. PMID: 10836817 No abstract available.
-
Comparison of St John's Wort and imipramine. Remission is important outcome.BMJ. 2001 Feb 24;322(7284):493; author reply 494. BMJ. 2001. PMID: 11222430 Free PMC article. No abstract available.
References
-
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders: depression and mania. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman's the pharmacological basis of therapeutics, 9th ed. New York: McGraw-Hill; 1996. pp. 431–459.
-
- Lépine JP, Gastpar M, Mendlewicz J, Tylee A.on behalf of the Depression Research in European Society Steering Committee. Depression in the community: the first pan-European study DEPRES (depression research in European society) Int Clin Psychopharmacol 19971219–29. - PubMed
-
- Linden M, Maier W, Achberger M, Herr R, Helmchen H, Benkert O. Psychiatric diseases and their treatment in general practice in Germany. Results of a World Health Organization (WHO) study. Nervenarzt. 1996;67:205–215. - PubMed
-
- Linde K, Mulrow CD Cochrane Collaboration, editors. Cochrane Library. Issue 2. Oxford: Update Software; 1999. St John's wort for depression.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
