Genetic analysis of mouse embryonic stem cells bearing Msh3 and Msh2 single and compound mutations
- PMID: 10594017
- PMCID: PMC85070
- DOI: 10.1128/MCB.20.1.149-157.2000
Genetic analysis of mouse embryonic stem cells bearing Msh3 and Msh2 single and compound mutations
Abstract
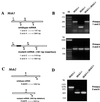
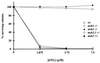
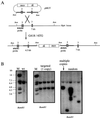
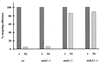
We have previously described the use of homologous recombination and CRE-loxP-mediated marker recycling to generate mouse embryonic stem (ES) cell lines homozygous for mutations at the Msh3, Msh2, and both Msh3 and Msh2 loci (2). In this study, we describe the analysis of these ES cells with respect to processes known to be affected by DNA mismatch repair. ES cells homozygous for the Msh2 mutation displayed increased resistance to killing by the cytotoxic drug 6-thioguanine (6TG), indicating that the 6TG cytotoxic mechanism is mediated by Msh2. The mutation rate of the herpes simplex virus thymidine kinase 1 (HSV-tk1) gene was unchanged in Msh3-deficient ES cell lines but markedly elevated in Msh2-deficient and Msh3 Msh2 double-mutant cells. Notably, the HSV-tk1 mutation rate was 11-fold higher, on average, than that of the hypoxanthine-guanine phosphoribosyl transferase (Hprt) locus in Msh2-deficient cells. Sequence analysis of HSV-tk1 mutants from these cells indicated the presence of a frameshift hotspot within the HSV-tk1 coding region. Msh3-deficient cells displayed a modest (16-fold) elevation in the instability of a dinucleotide repeat, whereas Msh2-deficient and Msh2 Msh3 double-mutant cells displayed markedly increased levels of repeat instability. Targeting frequencies of nonisogenic vectors were elevated in Msh2-deficient ES cell lines, confirming the role of Msh2 in blocking recombination between diverged sequences (homeologous recombination) in mammalian cells. These results are consistent with accumulating data from other laboratories and support the current model of DNA mismatch repair in mammalian cells.
Figures
Similar articles
-
Differential effects of the mismatch repair genes MSH2 and MSH3 on homeologous recombination in Saccharomyces cerevisiae.Mol Gen Genet. 1997 Dec;257(1):71-82. doi: 10.1007/pl00008619. Mol Gen Genet. 1997. PMID: 9439571
-
Mutation spectrum of MSH3-deficient HHUA/chr.2 cells reflects in vivo activity of the MSH3 gene product in mismatch repair.Mutat Res. 2000 Feb 14;447(2):155-64. doi: 10.1016/s0027-5107(99)00199-2. Mutat Res. 2000. PMID: 10751599
-
Mutator phenotype in Msh2-deficient murine embryonic fibroblasts.Cancer Res. 1997 Sep 1;57(17):3765-71. Cancer Res. 1997. PMID: 9288785
-
Genomic amplification of the human DHFR/MSH3 locus remodels mismatch recognition and repair activities.Adv Enzyme Regul. 1999;39:129-41. doi: 10.1016/s0065-2571(98)00013-2. Adv Enzyme Regul. 1999. PMID: 10470370 Review.
-
MutS homologs in mammalian cells.Curr Opin Genet Dev. 1997 Feb;7(1):105-13. doi: 10.1016/s0959-437x(97)80117-7. Curr Opin Genet Dev. 1997. PMID: 9024626 Review.
Cited by
-
Mechanisms maintaining genomic integrity in embryonic stem cells and induced pluripotent stem cells.Exp Biol Med (Maywood). 2011 Sep;236(9):987-96. doi: 10.1258/ebm.2011.011107. Epub 2011 Jul 18. Exp Biol Med (Maywood). 2011. PMID: 21768163 Free PMC article. Review.
-
Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library.Nat Biotechnol. 2014 Mar;32(3):267-73. doi: 10.1038/nbt.2800. Epub 2013 Dec 23. Nat Biotechnol. 2014. PMID: 24535568
-
Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage.Mol Cell Biol. 2002 Nov;22(22):7831-41. doi: 10.1128/MCB.22.22.7831-7841.2002. Mol Cell Biol. 2002. PMID: 12391152 Free PMC article.
-
Limitations of the Driver/Passenger Model in Cancer Prevention.Cancer Prev Res (Phila). 2016 May;9(5):335-8. doi: 10.1158/1940-6207.CAPR-15-0343. Epub 2016 Mar 1. Cancer Prev Res (Phila). 2016. PMID: 26932841 Free PMC article.
-
TREX2 deficiency suppresses spontaneous and genotoxin-associated mutagenesis.Cell Rep. 2024 Jan 23;43(1):113637. doi: 10.1016/j.celrep.2023.113637. Epub 2024 Jan 3. Cell Rep. 2024. PMID: 38175749 Free PMC article.
References
-
- Aaltonen L A, Peltomaki P, Leach F, Sistonen P, Pylkkanen S M, Mecklin J-P, Jarvinen H, Powell S, Jen J, Hamilton S R, Petersen G M, Kinzler K W, Vogelstein B, de la Chapelle A. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. - PubMed
-
- Barrett W L, First M R, Aron B S, Penn I. Clinical course of malignancies in renal transplant recipients. Cancer. 1993;72:2186–2189. - PubMed
-
- Bhattacharyya N P, Ganesh A, Phear G, Richards B, Skandalis A, Meuth M. Molecular analysis of mutations in mutator colorectal carcinoma cell lines. Hum Mol Genet. 1995;4:2057–2064. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
