Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells
- PMID: 10594018
- PMCID: PMC85071
- DOI: 10.1128/MCB.20.1.158-172.2000
Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells
Abstract
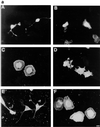
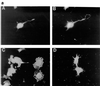
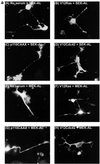
Ras and Rho family GTPases have been ascribed important roles in signalling pathways determining cellular morphology and growth. Here we investigated the roles of the GTPases Ras, Cdc42, Rac1, and Rho and that of phosphatidylinositol 3-kinase (PI 3-kinase) in the pathway leading from serum starvation to neurite outgrowth in N1E-115 neuroblastoma cells. Serum-starved cells grown on a laminin matrix exhibited integrin-dependent neurite outgrowth. Expression of dominant negative mutants of Ras, PI 3-kinase, Cdc42, or Rac1 all blocked this neurite outgrowth, while constitutively activated mutants of Ras, PI 3-kinase, or Cdc42 were each sufficient to promote outgrowth even in the presence of serum. A Ras(H40C;G12V) double mutant which binds preferentially to PI 3-kinase also promoted neurite formation. Activated Ras(G12V)-induced outgrowth required PI 3-kinase activity, but activated PI 3-kinase-induced outgrowth did not require Ras activity. Although activated Rac1 by itself did not induce neurites, neurite outgrowth induced by activated Cdc42(G12V) was Rac1 dependent. Cdc42(G12V)-induced neurites appeared to lose their normal polarization, almost doubling the average number of neurites produced by a single cell. Outgrowth induced by activated Ras or PI 3-kinase required both Cdc42 and Rac1 activity, but Cdc42(G12V)-induced outgrowth did not need Ras or PI 3-kinase activity. Active Rho(G14V) reduced outgrowth promoted by Ras(G12V). Finally, expression of dominant negative Jun N-terminal kinase or extracellular signal-regulated kinase did not inhibit outgrowth, suggesting these pathways are not essential for this process. Our results suggest a hierarchy of signalling where Ras signals through PI 3-kinase to Cdc42 and Rac1 activation (and Rho inactivation), culminating in neurite outgrowth. Thus, in the absence of serum factors, Ras may initiate cell cycle arrest and terminal differentiation in N1E-115 neuroblastoma cells.
Figures







Similar articles
-
NGF-dependent formation of ruffles in PC12D cells required a different pathway from that for neurite outgrowth.Neurochem Int. 2007 Jul-Sep;51(2-4):216-26. doi: 10.1016/j.neuint.2007.04.032. Epub 2007 May 17. Neurochem Int. 2007. PMID: 17561310
-
Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells.Mol Biol Cell. 2005 May;16(5):2207-17. doi: 10.1091/mbc.e04-10-0904. Epub 2005 Feb 23. Mol Biol Cell. 2005. PMID: 15728722 Free PMC article.
-
Integrin-linked kinase controls neurite outgrowth in N1E-115 neuroblastoma cells.J Biol Chem. 2001 Nov 16;276(46):42994-3003. doi: 10.1074/jbc.M105198200. Epub 2001 Sep 17. J Biol Chem. 2001. PMID: 11560928
-
The role of Rho GTPases and associated kinases in regulating neurite outgrowth.Int J Biochem Cell Biol. 2002 Jul;34(7):731-45. doi: 10.1016/s1357-2725(01)00167-4. Int J Biochem Cell Biol. 2002. PMID: 11950591 Review.
-
New model for the interaction of IQGAP1 with CDC42 and RAC1.Small GTPases. 2020 Jan;11(1):16-22. doi: 10.1080/21541248.2017.1321169. Epub 2017 Jun 19. Small GTPases. 2020. PMID: 28622072 Free PMC article. Review.
Cited by
-
PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells.Mol Cell Biol. 2002 Jan;22(2):567-77. doi: 10.1128/MCB.22.2.567-577.2002. Mol Cell Biol. 2002. PMID: 11756552 Free PMC article.
-
Oxidative stress induces tau hyperphosphorylation via MARK activation in neuroblastoma N1E-115 cells.J Clin Biochem Nutr. 2023 Jul;73(1):24-33. doi: 10.3164/jcbn.22-39. Epub 2023 May 16. J Clin Biochem Nutr. 2023. PMID: 37534088 Free PMC article.
-
PAK4 kinase is essential for embryonic viability and for proper neuronal development.Mol Cell Biol. 2003 Oct;23(20):7122-33. doi: 10.1128/MCB.23.20.7122-7133.2003. Mol Cell Biol. 2003. PMID: 14517283 Free PMC article.
-
CSK controls retinoic acid receptor (RAR) signaling: a RAR-c-SRC signaling axis is required for neuritogenic differentiation.Mol Cell Biol. 2007 Jun;27(11):4179-97. doi: 10.1128/MCB.01352-06. Epub 2007 Feb 26. Mol Cell Biol. 2007. PMID: 17325034 Free PMC article.
-
Cdc42 and RhoA reveal different spatio-temporal dynamics upon local stimulation with Semaphorin-3A.Front Cell Neurosci. 2015 Aug 26;9:333. doi: 10.3389/fncel.2015.00333. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26379503 Free PMC article.
References
-
- Barbacid M. Ras genes. Annu Rev Biochem. 1987;56:779–827. - PubMed
-
- Bar-Sagi D, Feramisco J R. Microinjection of the ras oncoprotein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. - PubMed
-
- Bar-Sagi D, Feramisco J R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. - PubMed
-
- Bollag G, McCormick F. Regulators and effectors of Ras proteins. Annu Rev Cell Biol. 1991;7:601–632. - PubMed
-
- Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
