Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes
- PMID: 10594043
- PMCID: PMC85096
- DOI: 10.1128/MCB.20.1.416-427.2000
Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes
Abstract
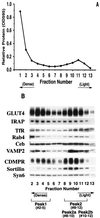
Insulin regulates glucose transport in muscle and adipose tissue by triggering the translocation of a facilitative glucose transporter, GLUT4, from an intracellular compartment to the cell surface. It has previously been suggested that GLUT4 is segregated between endosomes, the trans-Golgi network (TGN), and a postendosomal storage compartment. The aim of the present study was to isolate the GLUT4 storage compartment in order to determine the relationship of this compartment to other organelles, its components, and its presence in different cell types. A crude intracellular membrane fraction was prepared from 3T3-L1 adipocytes and subjected to iodixanol equilibrium sedimentation analysis. Two distinct GLUT4-containing vesicle peaks were resolved by this procedure. The lighter of the two peaks (peak 2) was comprised of two overlapping peaks: peak 2b contained recycling endosomal markers such as the transferrin receptor (TfR), cellubrevin, and Rab4, and peak 2a was enriched in TGN markers (syntaxin 6, the cation-dependent mannose 6-phosphate receptor, sortilin, and sialyltransferase). Peak 1 contained a significant proportion of GLUT4 with a smaller but significant amount of cellubrevin and relatively little TfR. In agreement with these data, internalized transferrin (Tf) accumulated in peak 2 but not peak 1. There was a quantitatively greater loss of GLUT4 from peak 1 than from peak 2 in response to insulin stimulation. These data, combined with the observation that GLUT4 became more sensitive to ablation with Tf-horseradish peroxidase following insulin treatment, suggest that the vesicles enriched in peak 1 are highly insulin responsive. Iodixanol gradient analysis of membranes isolated from other cell types indicated that a substantial proportion of GLUT4 was targeted to peak 1 in skeletal muscle, whereas in CHO cells most of the GLUT4 was targeted to peak 2. These results indicate that in insulin-sensitive cells GLUT4 is targeted to a subpopulation of vesicles that appear, based on their protein composition, to be a derivative of the endosome. We suggest that the biogenesis of this compartment may mediate withdrawal of GLUT4 from the recycling system and provide the basis for the marked insulin responsiveness of GLUT4 that is unique to muscle and adipocytes.
Figures







References
-
- Birnbaum M J, James D E. The insulin-regulatable glucose transporter GLUT-4. Curr Opin Endocrinol Diabetes. 1995;2:383–391.
-
- Cushman S W, Wardzala L J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell: apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
